Abstract
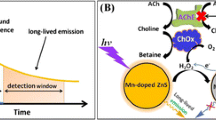
The authors describe a fluorometric "off-on-off" method for the determination of the activity of acetylcholine esterase (AChE). Molybdenum oxide quantum dots (QDs) are used as a fluorescent probe having excitation/emission peaks at 405/525 nm. It is found that the photoluminescence of such QDs is quenched by Cu(II) ion but that it can be restored by acetylcholine which is formed by AChE-catalyzed hydrolysis of acetylthiocholine. The effect is due to the strong affinity between Cu(II) and the thiol group of acetylthiocholine. Hence, quenching is increasingly reversed with increasing concentrations of AChE. However, fluorescence is not restored if the activity of AChE is inhibited by an inhibitor. Under optimal conditions, a linear relationship is founded in the 0.05 to 15 mU·mL−1 AChE activity range, with a limit of detection of 35 μU·mL−1 (applying the 3σ/k criterion). Three organophosphate pesticides and propazine were used to validate the assay. All showed strong inhibition, with IC 50 values (the concentration required for 50% inhibition to occur) to be 52, 221, 48 and 9 nM for diazinon, chlorpyriphos, monocrotophos and propazine. Benefitting from its sensitivity, specificity and simplicity, the assay in our perception provides a valuable tool for detection of AChE activity and in screening for its inhibitors.

A novel “off-on-off” assay is constructed for acetylcholine esterase (AChE) activity and inhibitor screening benefitting from the phenomenon that the photoluminescence of molybdenum oxide quantum dots (MoOx QDs) is quenched by Cu2+ and that the quenched photoluminescence can be restored due the strong affinity between Cu2+ and thiocholine (Tch) which is the hydrolysis product formed due to catalysis by AChE.






Similar content being viewed by others
References
Pavlov V, Xiao Y, Willner I (2005) Inhibition of the Acetycholine esterase-stimulated growth of au nanoparticles: nanotechnology-based sensing of nerve gases. Nano Lett 5:649–653. https://doi.org/10.1021/nl050054c
Khaldi K, Sam S, Gouget-Laemmel AC, Villeneuve CHA, Moraillon FO et al (2015) Active acetylcholinesterase immobilization on a functionalized silicon surface. Langmuir 31:8421–8428. https://doi.org/10.1021/acs.langmuir.5b01928
Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11:315–335. https://doi.org/10.2174/1570159X11311030006
Nakamura S, Takemura M, Suenaga T, Akiguchi I, Kimura J et al (1992) Occurrence of acetylcholinesterase activity closely associated with amyloid β/A4 protein is not correlated with acetylcholinesterase-positive fiber density in amygdala of Alzheimer's disease. Acta Neuropathol 84:425–432. https://doi.org/10.1007/BF00227670
Chen A, Du D, Lin Y (2012) Highly sensitive and selective Immuno-capture/electrochemical assay of acetylcholinesterase activity in red blood cells: a biomarker of exposure to organophosphorus pesticides and nerve agents. Environ Sci Technol 46:1828–1833. https://doi.org/10.1021/es202689u
Hou T, Zhang L, Sun X, Li F (2016) Biphasic photoelectrochemical sensing strategy based on in situ formation of CdS quantum dots for highly sensitive detection of acetylcholinesterase activity and inhibition. Biosens Bioelectron 75:359–364. https://doi.org/10.1016/j.bios.2015.08.063
Wei M, Zeng G, Lu Q (2014) Determination of organophosphate pesticides using an acetylcholinesterase-based biosensor based on a boron-doped diamond electrode modified with gold nanoparticles and carbon spheres. Microchim Acta 181:121–127. https://doi.org/10.1007/s00604-013-1078-4
Zhou S, Chen H, Wu B, Ma C, Ye Y (2012) Sensitive determination of carbamates in fruit and vegetables by a combination of solid-phase extraction and dispersive liquid-liquid microextraction prior to HPLC. Microchim Acta 176:419–427. https://doi.org/10.1007/s00604-011-0735-8
Li Y, Bai H, Li C, Shi G (2011) Colorimetric assays for acetylcholinesterase activity and inhibitor screening based on the disassembly assembly of a water-soluble Polythiophene derivative. ACS Appll Mater Interfaces 3:1306–1313. https://doi.org/10.1021/am200101n
Cui K, Chen Z, Wang Z, Zhang G, Zhang D (2011) A naked-eye visible and fluorescence "turn-on" probe for acetyl-cholinesterase assay and thiols as well as imaging of living cells. Analyst 136:191–195. https://doi.org/10.1039/c0an00456a
Lu L, Xia Y (2015) Enzymatic reaction modulated gold Nanorod end-to-end self-assembly for ultrahigh sensitively colorimetric sensing of cholinesterase and organophosphate pesticides in human blood. Anal Chem 87:8584–8591. https://doi.org/10.1021/acs.analchem.5b02516
Wang M, Gu X, Zhang G, Zhang D, Zhu D (2009) Continuous colorimetric assay for acetylcholinesterase and inhibitor screening with gold nanoparticles. Langmuir 25:2504–2507. https://doi.org/10.1021/la803870v
Ni P, Sun Y, Dai H, Jiang S, Lu W et al (2016) Colorimetric determination of the activity of acetylcholinesterase and its inhibitors by exploiting the iodide-catalyzed oxidation of 3,3′,5,5′-tetramethylbenzidine by hydrogen peroxide. Microchim Acta 183:2589–2595. https://doi.org/10.1007/s00604-016-1874-8
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90. https://doi.org/10.1016/0006-2952(61)90145-9
Lei C, Wang Z, Nie Z, Deng H, Hu H et al (2015) Resurfaced fluorescent protein as a sensing platform for label-free detection of copper(II) ion and acetylcholinesterase activity. Anal Chem 87:1974–1980. https://doi.org/10.1021/ac504390e
Wang M, Gu X, Zhang G, Zhang D, Zhu D (2009) Convenient and continuous Fluorometric assay method for acetylcholinesterase and inhibitor screening based on the aggregation-induced emission. Anal Chem 81:4444–4449. https://doi.org/10.1021/ac9002722
Liao S, Han W, Ding H, Xie D, Tan H et al (2013) Modulated dye retention for the signal-on Fluorometric determination of acetylcholinesterase inhibitor. Anal Chem 85:4968–4973. https://doi.org/10.1021/ac400865t
Du D, Chen S, Song D, Li H, Chen X (2008) Development of acetylcholinesterase biosensor based on CdTe quantum dots/gold nanoparticles modified chitosan microspheres interface. Biosens Bioelectron 24:475–479. https://doi.org/10.1016/j.bios.2008.05.005
Rodrigues SSM, Ribeiro DSM, Soares JX, Passos MLC, Saraiva MLMFS et al (2017) Application of nanocrystalline CdTe quantum dots in chemical analysis: implementation of chemo-sensing schemes based on analyte-triggered photoluminescence modulation. Coord Chem Rev 330:127–143. https://doi.org/10.1016/j.ccr.2016.10.001
Liu D, Chen W, Wei J, Li X, Wang Z et al (2012) A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem 84:4185–4191. https://doi.org/10.1021/ac300545p
Yi Y, Zhu G, Liu C, Huang Y, Zhang Y et al (2013) A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal Chem 85:11464–11470. https://doi.org/10.1021/ac403257p
Li W, Li W, Hu Y, Xia Y, Shen Q et al (2013) A fluorometric assay for acetylcholinesterase activity and inhibitor detectionbased on DNA-templated copper/silver nanoclusters. Biosens Bioelectron 47:345–349. https://doi.org/10.1016/j.bios.2013.03.038
Li H, Sun C, Vijayaraghavan R, Zhou F, Zhang X et al (2016) Long lifetime photoluminescence in N, S co-doped carbon quantum dots from an ionic liquid and their applications in ultrasensitive detection of pesticides. Carbon 104:33–39. https://doi.org/10.1016/j.carbon.2016.03.040
Lv B, Wei M, Liu Y, Liu X, Wei W et al (2016) Ultrasensitive photometric and visual determination of organophosphorus pesticides based on the inhibition of enzyme-triggered formation of core-shell gold-silver nanoparticles. Microchim Acta 183:2941–2948. https://doi.org/10.1007/s00604-016-1939-8
Ni P, Sun Y, Jiang S, Lu W, Wang Y et al (2017) Label-free detection of acetylcholinesterase and its inhibitor based on the in situ formation of fluorescent copper nanoparticles. Sensors Actuators B 240:651–656. https://doi.org/10.1016/j.snb.2016.08.096
Zhang Y, Cai Y, Qi Z, Lu L, Qian Y (2013) DNA-templated silver nanoclusters for fluorescence turn-on assay of acetylcholinesterase activity. Anal Chem 85:8455–8461. https://doi.org/10.1021/ac401966d
Xiao SJ, Zhao XJ, Zuo J, Huang HQ, Zhang L (2016) Highly photoluminescent MoOx quantum dots: facile synthesis and application in off-on pi sensing in lake water samples. Anal Chim Acta 906:148–155. https://doi.org/10.1016/j.aca.2015.12.022
Xiao SJ, Zhao XJ, Hu PP, Chu ZJ, Huang CZ et al (2016) Highly Photoluminescent molybdenum oxide quantum dots: one-pot synthesis and application in 2,4,6-trinitrotoluene determination. ACS Appll Mater Interfaces 8:8184–8191. https://doi.org/10.1021/acsami.5b11316
Krishnan CV, Chen J, Burger C, Chu B (2006) Polymer-assisted growth of molybdenum oxide whiskers via a Sonochemical process. J Phys Chem B 110:20182–20188. https://doi.org/10.1021/jp063156f
Brown NMD, Cui N, McKinley A (1998) An XPS study of the surface modification of natural MoS2 following treatment in an RF-oxygen plasma. Appl Surf Sci 134:11–21. https://doi.org/10.1016/S0169-4332(98)00252-9
Garg A, Cartier CA, Bishop KJM, Velegol D (2016) Particle zeta potentials remain finite in saturated salt solutions. Langmuir 32:11837–11844. https://doi.org/10.1021/acs.langmuir.6b02824
Sshiri M, Navaei-Nigjeh M, Baeeri M, Rrahimifard M, Mahboudi H et al (2016) Blockage of both the extrinsic and intrinsic pathways of diazinon-induced apoptosis in PaTu cells by magnesium oxide and selenium nanoparticles. Int J Nanomedicine 11:6239–6250. https://doi.org/10.2147/IJN.S119680
Raghu P, Reddy TM, Swamyb BEK, Chandrashekar BN, Reddaiah K et al (2012) Development of AChE biosensor for the determination of methyl parathion and monocrotophos in water and fruit samples: a cyclic voltammetric study. J Electroanal Chem 665:76–82. https://doi.org/10.1016/j.jelechem.2011.11.020
Chen Y, Hl R, Liu N, Sai N, Liu X et al (2010) A fluoroimmunoassay based on quantum dot−streptavidin conjugate for the detection of chlorpyrifos. J Agric Food Chem 58:8895–8903. https://doi.org/10.1021/jf101778t
Ju KJ, Feng JX, Feng JJ, Zhang QL, Xu TQ, Wei J, Wang AJ (2015) Biosensor for pesticide triazophos based on its inhibition of acetylcholinesterase and using a glassy carbon electrode modified with coral-like gold nanostructures supported on reduced graphene oxide. Microchim Acta 182:2427–2434. https://doi.org/10.1007/s00604-015-1584-7
Schmidel AJ, Assmanna KL, Werlang CC, Bertoncelloa KT, Francescon F et al (2014) Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol Teratol 44:62–69. https://doi.org/10.1016/j.ntt.2014.05.006
Wang H, Shumei M, Zhang F, Wang H, Liu H et al (2015) Effects of atrazine on the development of neural system of zebrafish, Danio Rerio. Biomed Res Int 2015:976068. https://doi.org/10.1155/2015/976068
Acknowledgments
This work has been supported by the National Natural Science-Foundation of China (No. 21465003, 11475044, 41461070 and 21561002), Natural Science Foundation of Jiangxi Province (No.20161BAB213090) and Jiangxi Provincial Department of education project (No. GJJ150583).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 520 kb)
Rights and permissions
About this article
Cite this article
Xiao, S.J., Chu, Z.J., Zhao, X.J. et al. Off-on-off detection of the activity of acetylcholine esterase and its inhibitors using MoOx quantum dots as a photoluminescent probe. Microchim Acta 184, 4853–4860 (2017). https://doi.org/10.1007/s00604-017-2519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2519-2