Abstract
The authors describe a voltammetric immunoassay for the carcinoembryonic antigen (CEA). A GCE was modified by electrodeposition of poly(3,4-ethylenedioxythiophene) (PEDOT) doped with tannic acid (TA). Subsequently, four-armed poly(ethylene glycol) (PEG) was assembled onto the modified surface through hydrogen bonding. The fabrication steps were characterized by scanning electron microscopy, energy dispersive spectroscopy, fourier transform infrared spectroscopy, contact angle measurements, electrochemical impedance spectroscopy and differential pulse voltammetry. The PEG/TA-PEDOT surface is shown be super-hydrophilic and to possess anti-fouling capability. Antibody against CEA was then covalently immobilized on the electrode. By using hexacyanoferrate as an electrochemical probe and at a working potential of 0.18 V vs SCE, the amperometric response is linear in the 10 ag·mL−1 to 1.0 ng·mL−1 CEA concentration range, and the detection limit is as low as 4.8 ag·mL−1 (at an S/N ratio of 3). The assay was applied to the quantification of CEA in 1:10 diluted human serum samples. Recoveries ranged from 103.7 to 108.7%, and relative standard deviations from 2.9 to 4.8%.

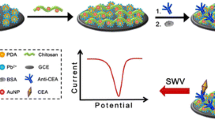
Schematic of an electrochemical immunosensor for the carcinoembryonic antigen (CEA). It is based on the use of tannic acid (TA) and poly(ethylene glycol) (PEG), both deposited on a glassy carbon electrode (GCE), and using hexacyanoferrate as the electrochemical probe. The sensor has a wide linear range and a 4.8 ag·mL−1 detection limit.








Similar content being viewed by others
References
Pan L, Zhao J, Huang Y, Zhao S, Liu YM (2015) Aptamer-based microchip electrophoresis assays for amplification detection of carcinoembryonic antigen. Clin Chim Acta 450:304–309
Gao J, Guo Z, Su F, Gao L, Pang X, Cao W, Du B, Wei Q (2015) Ultrasensitive electrochemical immunoassay for CEA through host-guest interaction of beta-cyclodextrin functionalized graphene and cu@Ag core-shell nanoparticles with adamantine-modified antibody. Biosens Bioelectron 63:465–471
Swierczewska M, Liu G, Lee S, Chen X (2012) High-sensitivity nanosensors for biomarker detection. Chem Soc Rev 41:2641–2655
Hasanzadeh M, Shadjou N, Lin Y, de la Guardia M (2017) Nanomaterials for use in immunosensing of carcinoembryonic antigen (CEA): recent advances. TrAC Trends Anal Chem 86:185–205
Hasanzadeh M, Shadjou N (2017) Advanced nanomaterials for use in electrochemical and optical immunoassays of carcinoembryonic antigen. A review. Microchim Acta 184:389–414
Sahiner N, Sagbas S, Aktas N (2015) Single step natural poly(tannic acid) particle preparation as multitalented biomaterial. Mater Sci Eng Proc Conf 49:824–834
Lin J, Yan F, Ju H (2004) Noncompetitive enzyme immunoassay for carcinoembryonic antigen by flow injection chemiluminescence. Clin Chim Acta 341:109–115
Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L (2003) The case for early detection. Nat Rev Cancer 3:243–252
Anderson NL, Anderson NG (2002) The human plasma proteome history, character, and diagnostic prospects. Mol Cell Proteomics 1:845–867
Brand RE, Nolen BM, Zeh HJ, Allen PJ, Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD, Vickers SM, Langmead CJ, Landsittle DP, Whitcomb DC, Grizzle WE, Lokshin AE (2011) Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res 17:805–816
Deng L, Mrksich M, Whitesides GM (1996) Self-assembled monolayers of alkanethiolates presenting tri (propylene sulfoxide) groups resist the adsorption of protein. J Am Chem Soc 118:5136–5137
Holmlin RE, Chen X, Chapman RG, Takayama ST, Whiteside GM (2001) Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 17:2841–2850
Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM (2001) A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 1718:5605–5620
Nowinski AK, Sun F, White AD, Keefe AJ, Jiang S (2012) Sequence, structure, and function of peptide self-assembled monolayers. J Am Chem Soc 134:6000–6005
Luo X, Xu M, Freeman C, James T, Davis JJ (2013) Ultrasensitive label free electrical detection of insulin in neat blood serum. Anal Chem 85:4129–4134
Garapaty A, Champion JA (2015) Non-covalent phosphorylcholine coating reduces protein adsorption and phagocytic uptake of microparticles. Chem Commun (Cambridge, U. K.) 51:13814–13817
Fan X, Su Y, Zhao X, Li Y, Zhang R, Ma T, Liu Y, Jiang Z (2016) Manipulating the segregation behavior of poly(ethylene glycol) by hydrogen bonding interaction to endow ultrafiltration membranes with enhanced antifouling performance. J Membr Sci 499:56–64
Pop-Georgievski O, Verreault D, Diesner MO, Proks V, Heissler S, Rypácek F, Koelsch P (2012) Non-fouling poly(ethylene oxide) layers end-tethered to Polydopamine. Langmuir 40:14273–14283
Shan J, Ma Z (2017) A review on amperometric immunoassays for tumor markers based on the use of hybrid materials consisting of conducting polymers and noble metal nanomaterials. Microchim Acta 184:969–979
Cho EC, Chang-Jian CW, Ho BC, Yu CC, Hsiao YS, Lee KC, Huang JH (2017) The effect of wetting property on electrochromic properties offunctionalize poly(3,4-ethylenedioxythiophene) films. Dyes Pigments 145:95–102
Chen S, Li L, Zhao C, Zheng J (2010) Surface hydration: principles and applications toward low-fouling/nonfouling biomaterials. Polymer 51:5283–5293
Yang Y, Liu Q, Liu Y, Cui J, Liu H, Wang P, Li Y, Chen L, Zhao Z, Dong Y (2017) A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens Bioelectron 90:31–38
Miao L, Jiao L, Zhang J, Li H (2017) Amperometric sandwich immunoassay for the carcinoembryonic antigen using a glassy carbon electrode modified with iridium nanoparticles, polydopamine and reduced graphene oxide. Microchim Acta 184:169–175
Yang T, Gao Y, Liu Z, Xu J, Lu L, Yu Y (2017) Three-dimensional gold nanoparticles/prussian blue-poly(3,4-ethylenedioxythiophene) nanocomposite as novel redox matrix for label-free electrochemical immunoassay of carcinoembryonic antigen. Sensors Actuators B Chem 239:76–84
Li X, Yu M, Chen Z, Lin X, Wu Q (2017) A sensor for detection of carcinoembryonic antigen based on the polyaniline-au nanoparticles and gap-based interdigitated electrode. Sensors Actuators B Chem 239:874–882
Yang Z, Lan Q, Li J, Wu J, Tang Y, Hu X (2017) Efficient streptavidin-functionalized nitrogen-doped graphene for the development of highly sensitive electrochemical immunosensor. Biosens Bioelectron 89:312–318
He B (2017) Differential pulse voltammetric assay for the carcinoembryonic antigen using a glassy carbon electrode modified with layered molybdenum selenide, graphene, and gold nanoparticles. Microchim Acta 184:229–235
Sun X, Hui N, Luo X (2017) Reagentless and label-free voltammetric immunosensor for carcinoembryonic antigen based on polyaniline nanowires grown on porous conducting polymer composite. Microchim Acta 184:889–896
Liu Q, Liu XP, Wei YP, Mao CJ, Niu HL, Song JM, Jin BK, Zhang SY (2017) Electrochemiluminescence immunoassay for the carcinoembryonic antigen using CdSe: Eu nanocrystals. Microchim Acta 184:1353–1360
Zhao D, Wang Y, Nie G (2016) Electrochemical immunosensor for the carcinoembryonic antigen based on a nanocomposite consisting of reduced graphene oxide, gold nanoparticles and poly (indole-6-carboxylic acid). Microchim Acta 183:2925–2932
Acknowledgements
This work was supported by the Joint Funds of the National Science Foundation of China (U1303283), National High Technology Research and Development Program of China (2015AA034602), China Postdoctoral Science Foundation (2015 M582738), The science and technology project and achievement transformation plan of modern agricultural of Xinjiang Corps (2016 AC010), The National Science and Technology Major Project (2017YFD0500304).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 941 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Lv, S., Gao, Z. et al. Voltammetric immunoassay for the carcinoembryonic antigen by using a glassy carbon electrode modified with poly(3,4-ethylenedioxythiophene) doped with tannic acid and grafted with poly(ethylene glycol). Microchim Acta 184, 4705–4712 (2017). https://doi.org/10.1007/s00604-017-2502-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2502-y