Abstract
A composite material consisting of multiwalled carbon nanotubes and palladium containing particles was synthesized and applied to the preparation of bulk-modified screen-printed carbon electrodes (Pd-MWCNT-SPCE) and surface-modified screen-printed carbon electrodes (Pd-MWCNT/SPCE). They were characterized by cyclic voltammetry and hydrodynamic chronoamperometry in solution of pH 7.5. Both electrodes were then modified with glucose oxidase (GOx) by drop-coating a solution of GOx and Nafion® on their surface. Glucose can be determined via enzymatically formed H2O2. In an alternative approach, gold nanoparticles (5 nm) were incorporated into the biolayer of the electrodes. The resulting electrodes of type GOx/Pd-MWCNT-SPCE and GOx-Au/Pd-MWCNT-SPCE showed acceptable analytical performance at working potentials between −0.20 V and −0.50 V in case of hydrodynamic chronoamperometry. Both electrodes can be operated best at a working potential of −0.40 V vs SCE, with acceptable linearity of the methods in sub mM concentration ranges and with LOQs of 0.14 mM and 0.07 mM for glucose for the GOx/Pd-MWCNT-SPCE and GOx-Au/Pd-MWCNT-SPCE, respectively. Incorporation of gold nanoparticles prolongs the operational lifetime of the electrodes by two weeks. The GOx/Pd-MWCNT-SPCE based method was applied to the determination of glucose in multifloral honey, and the GOx-Au/Pd-MWCNT-SPCE method to the determination of glucose in blood serum. In both cases there was a good agreement with the results obtained by commercially available equipment for determination of glucose.

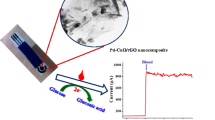
Schematic of a screen printed carbon biosensor based on the use of multiwalled carbon nanotubes modified with palladium-containing particles and glucose oxidase. It can be applied to the amperometric determination of glucose in blood serum and multifloral honey





Similar content being viewed by others
References
Clark LC (1979) The hydrogen peroxide sensing platinum anode as an analytical enzyme electrode. Methods Enzymol 56:448–479
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814–825
Taha Z, Wang J (1991) Electrocatalysis and flow detection at a glassy carbon electrode modified with a thin film of oxymanganese species. Electroanalysis 3:215–219
Beyene WN, Kotzian P, Schachl K, Alemu H, Turkušić E, Chopra A, Moderegger H, Švancara I, Vytřas K, Kalcher K (2004) (Bio)sensors based on manganese dioxide-modified carbon substrates: retrospections, further improvements and applications. Talanta 64:1151–1159
Turkušić E, Kalcher K, Schachl K, Komersová A, Bartoš M, Moderegger H, Švancara I, Vytřas K (2001) Amperometric determination of glucose with an MnO2 and glucose oxidase bulk-modified screen-printed carbon ink biosensor. Anal Lett 34:2633–2647
Turkusic E, Kalcher J, Kahrovic E, Beyene WN, Moderegger H, Sofic S, Begic E, Kalcher K (2005) Amperometric determination of bonded glucose with an MnO2 and glucose oxidase bulk-modified screen-printed electrode using flow-injection analysis. Talanta 65:559–564
Schachl K, Alemu H, Kalcher K, Moderegger H, Svancara I, Vytras K (1998) Amperometric determination of hydrogen peroxide with a manganese dioxide film-modified screen printed carbon electrode. Fresenius J Anal Chem 362:194–200
Zbiljić J, Guzsvány V, Vajdle O, Prlina B, Agbaba J, Dalmacija B, Kónya Z, Kalcher K (2015) Determination of H2O2 by MnO2 modified screen printed carbon electrode during Fenton and visible light-assisted photo-Fenton based removal of acetamiprid from water. J Electroanal Chem 755:77–86
Turkusic E, Begic S, Kahrovic E, Kalcher K (2011) Amperometric determination of hydrogen peroxide with FeO bulk-modified screen-printed carbon ink sensor. Health MED 5:949–955
Erden PE, Zeybek B, Pekyardimc Ş, Kiliç E (2013) Amperometric carbon paste enzyme electrodes with Fe3O4 nanoparticles and 1,4-benzoquinone for glucose determination. Artif Cells Nanomed Biotechnol 41:165–171
Baratella D, Magro M, Sinigaglia G, Zboril R, Salviulo G, Vianello F (2013) A glucose biosensor based on surface active maghemite nanoparticles. Biosens Bioelectron 45:13–18
Zhang L, Ni Y, Wang X, Zhao G (2010) Direct electrocatalytic oxidation of nitric oxide and reduction of hydrogen peroxide based on α-Fe2O3 nanoparticles-chitosan composite. Talanta 82:196–201
Kotzian P, Brázdilová P, Kalcher K, Handlíř K, Vytřas K (2007) Oxides of platinum metal group as potential catalysts in carbonaceous amperometric biosensors based on oxidases. Sensors Actuators B Chem 124:297–302
Kotzian P, Brázdilová P, Kalcher K, Vytřas K (2005) Determination of hydrogen peroxide, glucose and hypoxanthine using (bio)sensors based on ruthenium dioxide-modified screen printed electrodes. Anal Lett 38:1099–1113
Kotzian P, Brázdilová P, Rezková S, Kalcher K, Vytřas K (2006) Amperometric glucose biosensor based on rhodium dioxide-modified carbon ink. Electroanalysis 18:1499–1504
Karyakin AA (2001) Prussian blue and its analogues: electrochemistry and analytical applications. Electroanalysis 13:813–819
Ricci F, Palleschi G (2005) Sensor and biosensor preparation, optimisation and applications of Prussian blue modified electrodes. Biosens Bioelectron 21:389–407
Karyakin AA, Puganova EA, Bolshakov IA, Karyakina EE (2007) Electrochemical sensor with record performance characteristics. Angew Chem Int Ed 46:7678–7680
Lin J, Zhou DM, Hocevar SB, McAdams ET, Ogorevc B, Zhang X (2005) Nickel hexacyanoferrate modified screen-printed carbon electrode for sensitive detection of ascorbic acid and hydrogen peroxide. Front Biosci 10:483–491
Kotzian P, Janku T, Kalcher K, Vytřas K (2007) Catalytic activity of iron hexacyanoosmate(II) towards hydrogen peroxide and nicotinamide adenine dinucleotide and its use in amperometric biosensors. Anal Chim Acta 599:287–293
Merkoçi A, Pumera M, Llopis X, Pérez B, del Valle M, Alegret S (2005) New materials for electrochemical sensing VI: carbon nanotubes. Trends Anal Chem 24:826–838
Gooding JJ (2005) Nanostructuring electrodes with carbon nanotubes: a review on electrochemistry and applications for sensing. Electrochim Acta 50:3049–3060
Guldi DM, Rahman GMA, Zerbetto F, Prato M (2005) Carbon nanotubes in electron donor - acceptor nanocomposites. Acc Chem Res 38:871–878
Daniel S, Rao TP, Rao KS, Rani SU, Naidu GRK, Lee HY, Kawai T (2007) A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sensors Actuators B Chem 122:672–682
Lin Y, Taylor S, Li HP, Fernando KAS, Qu LW, Wang W, Gu LR, Zhou B, Sun YP (2004) Advances toward bioapplication of carbon nanotubes. J Mater Chem 14:527–541
Callegari A, Cosnier S, Marcaccio M, Paolucci D, Paolucci F, Georgakilas V, Tagmatarchis N, Vasquez E, Prato M (2004) Functionalised single wall carbon nanotubes/polypyrrole composites for the preparation of amperometric glucose biosensors. J Mater Chem 14:807–810
Yu X, Chattopadhyay D, Galeska I, Papadimitrakopoulos F, Rusling JF (2003) Peroxidase activity of enzymes bound to the end of single wall carbon nanotube forest electrodes. Electrochem Commun 5:408–411
Lin Y, Lu F, Tu Y, Ren Z (2004) Glucose biosensor based on carbon nanotube nanoelectrode ensembles. Nano Lett 4:191–195
Dai YQ, Shiu KK (2004) Glucose biosensor based on multi-walled carbon nanotube modified glassy carbon electrode. Electroanalysis 16:1697–1703
Yao Y, Shiu KK (2007) Electron transfer properties of different carbon nanotube materials and their applications in glucose biosensors. Anal Bioanal Chem 387:303–309
Xu Y, Pehrsson PE, Chen L, Zhang R, Zhao W (2007) Double-stranded DNA single-walled carbon nanotube hybrids for optical hydrogen peroxide and glucose sensing. J Phys Chem C 111:8638–8643
Guiseppi-Elie A, Lei C, Baughman R (2002) Direct electron transfer of glucose oxidase on carbon nanotubes. Nanotechnology 13:559–564
Wildgoose GG, Banks CE, Compton RG (2006) Metal nanoparticles and related materials supported on carbon nanotubes: methods and applications. Small 2:182–193
Xue B, Chen P, Hong Q, Lin J, Tan KL (2001) Growth of Pd, Pt, Ag and Au nanoparticles on carbon nanotubes. J Mater Chem 11:2378–2381
Wang J (2005) Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17:7–14
Norouzi P, Faridbod F, Larijani B, Ganjali RM (2010) Glucose biosensor based on MWCNTs-gold nanoparticles in a nafion film on a glassy carbon electrode using flow injection FFT continuous cyclic voltammetry. Int J Electrochem Sci 5:1213–1224
You J-M, Jeon S (2011) A glassy carbon electrode modified with glucose oxidase and MWCNT-palladium nanoparticles for the determination of glucose. Electroanalysis 23:2103–2108
Hrapovic S, Liu YL, Male KB, Luong JHT (2004) Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal Chem 76:1083–1088
Yang J, Zhang RY, Xu Y, He PG, Fang YZ (2008) Direct electrochemistry study of glucose oxidase on Pt nanoparticle-modified aligned carbon nanotubes electrode by the assistance of chitosan-CdS and its biosensoring for glucose. Electrochem Commun 10:1889–1892
Wang J, Naser N, Angnes L, Wu H, Chen L (1992) Metal-dispersed carbon paste electrodes. Anal Chem 64:1285–1288
Zhang J, Li J, Yang F, Zhang B, Yang X (2009) Preparation of prussian blue@Pt nanoparticles/carbon nanotubes composite material for efficient determination of H2O2. Sensors Actuators B Chem 143:373–380
Guzmán C, Orozco G, Verde Y, Jiménez S, Godínez LA, Juaristi E, Bustos E (2009) Hydrogen peroxide sensor based on modified vitreous carbon with multiwall carbon nanotubes and composites of Pt nanoparticles–dopamine. Electrochim Acta 54:1728–1732
You J-M, Jeong NY, Ahmed SM, Kim KS, Choi CH, Jeon S (2011) Fabrication and application of amperometric glucose biosensor based on a novel PtPd bimetallic nanoparticle decorated multi-walled carbon nanotube catalyst. Biosens Bioelectron 26:2287–2291
Kang X, Mai Z, Zou X, Cai P, Mo J (2007) A novel glucose biosensor based on immobilization of glucose oxidase in chitosan on a glassy carbon electrode modified with gold-platinum alloy nanoparticles/multiwall carbon nanotubes. Anal Biochem 369:71–79
Ye Y, Ding S, Ye Y, Xu H, Cao X, Liu S, Sun H (2015) Enzyme-based sensing of glucose using a glassy carbon electrode modified with a one-pot synthesized nanocomposite consisting of chitosan, reduced graphene oxide and gold nanoparticles. Microchim Acta 182:1783–1789
Wang F, Gong W, Wang L, Chen Z (2015) Enhanced amperometric response of a glucose oxidase and horseradish peroxidase based bienzyme glucose biosensor modified with a film of polymerized toluidine blue containing reduced graphene oxide. Microchim Acta 182:1949–1956
Devasenathipathy R, Karthik R, Chen SM, Ali MA, Mani V, Lou BS, Al-Hemaid FMA (2015) Enzymatic glucose biosensor based on bismuth nanoribbons electrochemically deposited on reduced graphene oxide. Microchim Acta 182:2165–2172
Li Z, Sheng L, Meng A, Xie C, Zhao K (2016) A glassy carbon electrode modified with a composite consisting of reduced graphene oxide, zinc oxide and silver nanoparticles in a chitosan matrix for studying the direct electron transfer of glucose oxidase and for enzymatic sensing of glucose. Microchim Acta 183:1625–1632
Aslan S, Anik Ü (2016) Microbial glucose biosensors based on glassy carbon paste electrodes modified with Gluconobacter Oxydans and graphene oxide or graphene-platinum hybrid nanoparticles. Microchim Acta 183:73–81
Nayak P, Nair SP, Ramaprabhu S (2016) Enzyme-less and low-potential sensing of glucose using a glassy carbon electrode modified with palladium nanoparticles deposited on graphene-wrapped carbon nanotubes. Microchim Acta 183:1055–1062
Li J, Lu M, Tan Z, Xu Y, Zhang Y, Hu X, Yang Z (2016) One-step solvothermal preparation of silver-ZnO hybrid nanorods for use in enzymatic and direct electron-transfer based biosensing of glucose. Microchim Acta 183:1705–1712
Tangkuaram T, Ponchio C, Kangkasomboon T, Katikawong P, Veerasai W (2007) Design and development of a highly stable hydrogen peroxide biosensor on screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan. Biosens Bioelectron 22:2071–2078
Kang X, Mai Z, Zou X, Cai P, Mo J (2007) Electrochemical biosensor based on multi-walled carbon nanotubes and Au nanoparticles synthesized in chitosan. J Nanosci Nanotechnol 7:1618–1624
Wang J, Musameh M, Lin YH (2003) Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J Am Chem Soc 125:2408–2409
Yuan CJ, Wang YC, Reiko O (2009) Improving the detection of hydrogen peroxide of screen-printed carbon paste electrodes by modifying with nonionic surfactants. Anal Chim Acta 653:71–76
Kalcher K, Švancara I, Metelka R, Vytras K, Walcarius A (2006) Heterogeneous carbon electrochemical sensors. In: Grimes CA, Dickey EC, Pishko MV (eds) Encyclopedia of sensors, American scientific publishers, vol 4. American Scientific Publishers, Stevenson Ranch, pp 283–430
Hart JP, Wring SA (1994) Screen-printed voltammetric and amperometric electrochemical sensors for decentralized testing. Electroanalysis 6:617–624
P O’Halloran M, Pravda M, Guilbault GG (2001) Prussian blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta 55:3605–3611
Cinti S, Arduini F, Moscone D, Palleschi G, Killard JA (2014) Development of a hydrogen peroxide sensor based on screen-printed electrodes modified with inkjet-printed prussian blue nanoparticles. Sensors 14:14222–14234
Niesz K, Siska A, Vesselényi I, Hernadi K, Méhn D, Galbács G, Kónya Z, Kiricsi I (2002) Mechanical and chemical breaking of multiwalled carbon nanotubes. Catal Today 76:3–10
Veseli A, Hajrizi A, Arbneshi T, Kalcher K (2012) A new amperometric glucose biosensor based on screen printed carbon electrodes with rhenium (IV)-oxide as mediator. J Electrochem Sci Eng 2:199–210
Anojčić J, Guzsány V, Vajdle O, Madarász D, Rónavári A, Kónya Z, Kalcher K (2016) Hydrodynamic chronoamperometric determination of hydrogen peroxide using carbon paste electrodes coated by multiwalled carbon nanotubes decorated with MnO2 or Pt particles. Sensors Actuators B Chem 233:83–92
Berisha L, Kalcher K, Hajrizi A, Arbneshi T (2013) A new biosensor for glucose based on screen printed carbon electrodes modified with tin(IV)-oxide. AJAC 4:27–35
Wang J, Pedrero M, Pamidi PVA, Cai X (1995) Metal-dispersed screen-printed carbon electrodes. Electroanalysis 7:1032–1035
Acknowledgements
The authors acknowledge the financial support of the Ministry of Science and Technological Development of the Republic of Serbia (Project Nos. 172059 and 172012), and CEEPUSIII (CZ-0212-09-1516) network, and for MATCROSS (HUSRB 1002/214/188) for the synthesis of MWCNT and Pd-MWCNT materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Guzsvány, V., Anojčić, J., Radulović, E. et al. Screen-printed enzymatic glucose biosensor based on a composite made from multiwalled carbon nanotubes and palladium containing particles. Microchim Acta 184, 1987–1996 (2017). https://doi.org/10.1007/s00604-017-2188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2188-1