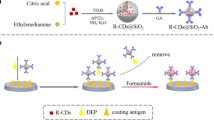
Abstract
Blue fluorescent carbon dots (C-Dots) were synthesized by a hydrothermal process using citric acid and polyethylene-polyamines as the precursors. The C-dots display strong fluorescence, excellent water solubility, good biocompatibility, and possess many amino groups on their surface. Biotin was linked to the amino groups, and the resulting biotinylated C-dots were applied in a binding assay where they compete with free biotin (vitamin B7; coenzyme R) for binding to streptavidin immobilized on superparamagnetic Dynabeads®. After incubation and magnetic separation, the fluorescence intensity of immobilized biotinylated C-dots released from the beads is inversely proportional to the concentration of biotin in the 0.5 to 100 ng·mL−1 concentration range. The detection limit is 0.1 ng·mL−1. Conceivably, the method may be further extended to the determination of biotinylated biomolecules.

One-pot hydrothermal synthesized C-Dots were biotinylated and used as fluorescent probe in the immunoassay for the determination of Vitamin B7.







Similar content being viewed by others
References
Lin Y, Yao B, Huang T, Zhang S, Cao X, Weng W (2016) Selective determination of free dissolved chlorine using nitrogen-doped carbon dots as a fluorescent probe. Microchim Acta 183(7):2221–2227
Li X, Zhang S, Kulinich SA, Liu Y, Zeng H (2014) Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci Rep 4:1–8
Yang ST, Cao L, Luo PG, Lu F, Wang X, Wang H, Meziani MJ, Liu Y, Qi G, Sun YP (2009) Carbon dots for optical imaging in vivo. J Am Chem Soc 131:11308–11309
Yulong Y, Xinsheng P (2016) Recent advances in carbon-based dots for electroanalysis. Analyst 141:2619–2628
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Wang W, Lu YC, Huang H, Feng JJ, Chen JR, Wang AJ (2014) Facile synthesis of water-soluble and biocompatible fluorescent nitrogen-doped carbon dots for cell imaging. Analyst 139:1692–1696
Hola K, Zhang Y, Wang Y, Giannelis EP, Zboril R, Rogach AL (2014) Carbon dots-emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9:590–603
Najafabadi AT, Gyenge E (2014) High-yield graphene production by electrochemical exfoliation of graphite: novel ionic liquid (IL)–acetonitrile electrolyte with low IL content. Carbon 71:58–69
Gonçalves H, Jorge PAS, Fernandes JRA, Esteves da Silva JCG (2010) Hg(II) sensing based on functionalized carbon dots obtained by direct laser ablation. Sensor Actuat B-Chem 145:702–707
Jiang Y (2015) N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+ detection. New J Chem 39:3357–3360
Wu Y, Wei P, Pengpumkiat S, Schumacher EA, Remcho VT (2015) Development of a carbon dot (C-dot)-linked immunosorbent assay for the detection of human α-fetoprotein. Anal Chem 87:8510–8516
Liu H, Wang Q, Shen G, Zhang C, Li C, Ji W, Wang C, Cui D (2014) A multifunctional ribonuclease A-conjugated carbon dot cluster nanosystem for synchronous cancer imaging and therapy. Nanoscale Res Lett 9:1–11
Mohapatra S, Sahu S, Sinha N, Bhutia SK (2014) Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg(2+) in water and living cells. Analyst 140:1221–1228
Yuan Y, Liu Z, Li R, Zou H, Lin M, Liu H, Huang C (2016) Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale 8:294–297
Bailey LM, Ivanov RA, Wallace JC, Polyak SW (2008) Artifactual detection of biotin on histones by streptavidin. Anal Biochem 373:71–77
Otten JJ, Hellwig JP, Meyers LD, Medicine IO (2006) DRI, dietary reference intakes: the essential guide to nutrient requirements. Am J Clin Nutr
Huang JL, Yang ZW (2015) Microorganism determination of biotin content in multi-vitamins tablets. J Food Saf Qual 6(8):3045–3049
Thompson LB, Schmitz DJ, Pan SJ (2006) Determination of biotin by high-performance liquid chromatography in infant formula, medical nutritional products, and vitamin premixes. J AOAC Int 89:1515–1518
Wang FL, Liu AG, Sun JJ, Peng C (2013) Simultaneous determination of folic acid, Vitamin B12 and biotin in infant formula by HPLC-MS-MS. Food Sci 34: 269–272
Sun MJ, Chen YS, Zhang XW, Chen Q (2014) Detection of biotin in milk powder basing on surface plasmon resonance. J Food Saf Qual 5:1724–1727
Schray KJ, Artz PG, Hevey RC (1988) Determination of avidin and biotin by fluorescence polarization. Anal Chem 60:853–855
Wilchek M, Bayer EA (1988) The avidin-biotin complex in bioanalytical applications. Anal Biochem 171:1–32
Wilchek M, Bayer EA (1984) The avidin-biotin complex in immunology. Immunol Today 5:39–43
Gajos K, Petrou P, Budkowski A, Awsiuk K, Bernasik A, Misiakos K, Rysz J, Raptis I, Kakabakos S (2014) Imaging and spectroscopic comparison of multi-step methods to form DNA arrays based on the biotin-streptavidin system. Analyst 140:1127–1139
Dundas CM, Demonte D, Park S (2013) Streptavidin-biotin technology: improvements and innovations in chemical and biological applications. Appl Microbiol Biotechnol 97:9343–9353
Stayton PS, Freitag S, Klumb LA, Chilkoti A, Chu V, Penzotti JE, To R, Hyre D, Trong IL, Lybrand TP (1999) Streptavidin–biotin binding energetics. Biomol Eng 16:39–44
Srisa-Art M, Dyson EC, Demello AJ, Edel JB (2008) Monitoring of real-time streptavidin-biotin binding kinetics using droplet microfluidics. Anal Chem 80:7063–7067
Wilson R (2011) Preparation of single-stranded DNA from PCR products with streptavidin magnetic beads. Nucleic Acid Ther 21:437–440
Björner M, Bergh A, Glad G, Granéer T, Hedlund H, Hellberg U, Uhléen K (2012) High performance protein enrichment using streptavidin magnetic beads. J Biomol Tech 23:S30
Anders H, Anna B, Olof N, Morten L, Joakim L, Mathias U (2005) The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 26:501–510
Acknowledgements
The authors gratefully acknowledge the financial support of National Natural Science Foundation of China (Nos. 21305015, Nos. 21275028), National Science Foundation for Distinguished Young Scholars of Fujian Province (Nos. 2016 J06019), Natural Science Foundation of Fujian Province of China (Nos. 2014 J05014, 2014 J07009), Joint Funds for the innovation of science and Technology(Fujian province, Nos. 2016Y91010007), Program for Fujian University Outstanding Youth Scientific Research (Nos. 2015b026), and Program for Fujian Top-notch Innovative Personnel.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests
Additional information
The first two authors Wensong Yao and Namei Wu contribute equally to the present manuscript.
Electronic supplementary material
ESM 1
(DOC 198 kb)
Rights and permissions
About this article
Cite this article
Yao, W., Wu, N., Lin, Z. et al. Fluorescent turn-off competitive immunoassay for biotin based on hydrothermally synthesized carbon dots. Microchim Acta 184, 907–914 (2017). https://doi.org/10.1007/s00604-017-2078-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2078-6