Abstract
We describe an electrochemical immunosensor for the determination of the growth promoter α-zearalanol in bovine serum. The sensing scheme is based on a nanocomposite consisting of gold nanoparticles electrodeposited on multi-walled carbon nanotubes that were modified with poly (vinylpyridine) through in-situ polymerization. The electrodeposition of the gold nanoparticles enlarges the surface available for immobilization of antibodies against α-zearalanol. The nanocomposite film was characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy, and cyclic voltammetry. The calibration plot has a linear response in the concentrations range from 0.05 to 50 ng mL−1, and the detection limit is 16 pg mL−1. The time required for analysis is 12 min only which compares quite favorably with the time (90 min) required by the conventional ELISA. The method exhibits good selectivity, stability and reproducibility for detecting α-zearalanol in the livestock production.

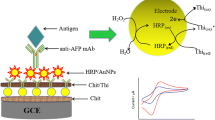
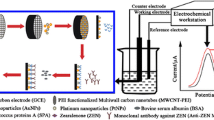
Schematic representation of the immunocapture procedure and subsequent determination of α-zearalanol.






Similar content being viewed by others
References
Bartelt-Hunt SL, Snow DD, Kranz WL, Mader TL, Shapiro CA, Van Donk SJ, Shelton DP, Tarkalson DD, Zhang TC (2012) Effect of growth promotants on the occurrence of endogenous and synthetic steroid hormones on feedlot soils and in runoff from beef cattle feeding operations. Environ Sci Technol 46:1352–1360
Wang YK, Yan YX, Mao ZW, Ha W, Zou Q, Hao QW, Ji WH, Sun JH (2013) Highly sensitive electrochemical immunoassay for zearalenone in grain and grain-based food. Microchim Acta 180:187–193
Blokland MH, Sterk SS, Stephany RW, Launay FM, Kennedy DG, Van Ginkel LA (2006) Determination of resorcylic acid lactones in biological samples by GC–MS. Discrimination between illegal use and contamination with fusarium toxins. Anal Bioanal Chem 384:1221–1227
Valenzuela-Grijalva NV, González-Rios H, Islava TY, Valenzuela M, Torrescano G, Camou JP, Núñez-González FA (2012) Changes in intramuscular fat, fatty acid profile and cholesterol content induced by zeranol implantation strategy in hair lambs. J Sci Food Agric 92:1362–1367
Matraszek-Zuchowska I, Wozniak B, Zmudzki J (2013) Determination of zeranol, taleranol, zearalanone, α-zearalenol, β-zearalenol and zearalenone in urine by LC-MS/MS. Food Addit Contam Part A-Chem 30:987–994
Roy JR, Chakraborty S, Chakraborty TR (2009) Estrogen-like endocrine disrupting chemicals affecting puberty in humans–A review. Med Sci Monitor 15:137–145
Council Directive 88/146/EEC (1988) Off J Eur Commun 70:16–18
Sánchez Arribas A, Bermejo E, Zapardiel A, Téllez H, Rodríguez-Flores J, Zougagh M, Ríos Á, Chicharro M (2009) Screening and confirmatory methods for the analysis of macrocyclic lactone mycotoxins by CE with amperometric detection. Electrophoresis 30:499–506
Han H, Kim B, Lee SG, Kim J (2013) An optimised method for the accurate determination of zeranol and diethylstilbestrol in animal tissues using isotope dilution-liquid chromatography/mass spectrometry. Food Chem 140:44–51
De Baere S, Osselaere A, Devreese M, Vanhaecke L, De Backer P, Croubels S (2012) Development of a liquid–chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Anal Chim Acta 756:37–48
Impens S, Van Loco J, Degroodt JM, De Brabander H (2007) A downscaled multi-residue strategy for detection of anabolic steroids in bovine urine using gas chromatography tandem mass spectrometry (GC–MS3). Anal Chim Acta 586:43–48
Välimaa AL, Kivistö AT, Leskinen PI, Karp MT (2010) A novel biosensor for the detection of zearalenone family mycotoxins in milk. J Microbiol Methods 80:44–48
Zhu J, Tao X, Ding S, Shen J, Wang Z, Wang Y, Xu F, Wu X, Hu T, Zhu A, Jiang H (2012) Micro-plate chemiluminescence enzyme immunoassay for determination of zeranol in bovine milk and urine. Anal Lett 45:2538–2548
Feng R, Zhang Y, Yu H, Wu D, Ma H, Zhu B, Xu C, Li H, Du B, Wei Q (2013) Nanoporous PtCo-based ultrasensitive enzyme-free immunosensor for Zeranol detection. Biosens Bioelectron 42:367–372
Feng R, Zhang Y, Li H, Wu D, Xin X, Zhang S, Yu H, Wei Q, Du B (2013) Ultrasensitive electrochemical immunosensor for zeranol detection based on signal amplification strategy of nanoporous gold films and nano-montmorillonite as labels. Anal Chim Acta 758:72–79
Xue X, Wei D, Feng R, Wang H, Wei Q, Du B (2013) Label-free electrochemical immunosensors for the detection of zeranol using graphene sheets and nickel hexacyanoferrate nanocomposites. Anal Methods 5:4159–4164
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2008–2013) Screen-printed electrodes for biosensing: a review. Microchim Acta
Ijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Pompeo F, Resasco DE (2002) Water solubilization of single-walled carbon nanotubes by functionalization with glucosamine. Nano Lett 4:369–373
Shamsipur M, Najafi M, Hosseini MRM (2010) Highly improved electrooxidation of glucose at a nickel (II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Biogeosciences 77:120–124
Qin S, Qin D, Ford WT, Resasco DE, Herrera JE (2004) Functionalization of single-walled carbon nanotubes with polystyrene via grafting to and grafting from methods. Macromolecules 37:752–757
Silva CCeC, Breitkreitz MC, Santhiago M, Crispilho Corrêa C, Tatsuo Kubota L (2012) Construction of a new functional platform by grafting poly (4-vinylpyridine) in multi-walled carbon nanotubes for complexing copper ions aiming the amperometric detection of l-cysteine. Electrochim Acta 71:150–158
Banerjee S, Benny TH, Wong SS (2005) Covalent surface chemistry of single-walled carbon nanotubes. Adv Mater 17:17–29
Sahoo NG, Rana S, Cho JW, Li L, Chan SH (2010) Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci 35:837–867
Wang J (2012) Electrochemical biosensing based on noble metal nanoparticles. Microchim Acta 177:245–270
Pereira SV, Bertolino FA, Messina GA, Raba J (2011) Microfluidic immunosensor with gold nanoparticle platform for the determination of immunoglobulin G anti-Echinococcus granulosus antibodies. Anal Biochem 409:98–104
Cai X, Gao X, Wang L, Wu Q, Lin X (2013) A layer-by-layer assembled and carbon nanotubes/gold nanoparticles-based bienzyme biosensor for cholesterol detection. Sens Actuator B-Chem 181:575–583
Batra B, Pundir CS (2013) An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/ gold nanoparticles/chitosan composite film modified Au electrode. Biosens Bioelectron 47:496–501
Acknowledgments
The authors wish to thank the financial support from Universidad Nacional de San Luis (UNSL), Instituto de Química de San Luis (INQUISAL), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 66 kb)
Rights and permissions
About this article
Cite this article
Regiart, M., Seia, M.A., Messina, G.A. et al. Electrochemical immunosensing using a nanostructured functional platform for determination of α-zearalanol. Microchim Acta 182, 531–538 (2015). https://doi.org/10.1007/s00604-014-1355-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1355-x