Abstract
Purpose
Given that left upper lobe and right upper and middle lobes share a similar anatomy, segmentectomy, such as upper division and lingulectomy, should yield identical oncological clearance to left upper lobectomy. We compared the prognosis of segmentectomy with that of lobectomy for early stage non-small-cell lung cancer (NSCLC) in the left upper lobe.
Methods
We retrospectively examined 2115 patients who underwent segmentectomy or lobectomy for c-stage I (TNM 8th edition) NSCLC in the left upper lobe in 2010. We compared the oncological outcomes of segmentectomy (n = 483) and lobectomy (n = 483) using a propensity score matching analysis.
Results
The 5-year recurrence-free and overall survival rates in the segmentectomy and lobectomy groups were comparable, irrespective of c-stage IA or IB. Subset analyses according to radiological tumor findings showed that segmentectomy yielded oncological outcomes comparable to those of lobectomy for non-pure solid tumors. In cases where the solid tumor exceeded 20 mm, segmentectomy showed a recurrence-free survival inferior to that of lobectomy (p = 0.028), despite an equivalent overall survival (p = 0.38).
Conclusion
Segmentectomy may be an acceptable alternative to lobectomy with regard to the overall survival of patients with c-stage I NSCLC in the left upper lobe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of segmentectomy has expanded to include resection of peripheral small-sized early non-small-cell lung cancer (NSCLC), as this technique may better preserve the lung function than lobectomy [1, 2]. Recently, a Japanese randomized controlled trial to confirm the non-inferiority of segmentectomy to lobectomy in patients with small peripheral NSCLC (JCOG0802/WJOG4607L) revealed a significantly better overall survival (OS) with segmentectomy than lobectomy, suggesting that it could become the standard treatment instead of lobectomy for this population [3]. Although segmentectomy exhibits a significantly higher rate of local relapse than lobectomy [4], this result may help us perform less-invasive resection of a smaller volume of lung tissue and facilitates tailor-made surgery for each patient.
As the left upper lobe is one of the largest lobes in the lungs, segmentectomies, such as upper division segmentectomy and lingulectomy, are well established as acceptable treatments in thoracic surgery. In particular, the anatomy of the left upper division (segments 1+2 and 3) and lingula (segments 4 and 5) is similar to that of the right upper and middle lobes. Several studies have demonstrated equal oncological outcomes between split lobectomy (upper division segmentectomy or lingulectomy) and left upper lobectomy [5,6,7,8,9]. We previously compared the oncological outcomes between upper division segmentectomy and lobectomy in the left upper division located in cN0 NSCLC and demonstrated that oncological outcomes following upper division segmentectomy were not inferior to those following lobectomy, even when the tumor was larger than 2 cm and located close to the intersegmental plane [10]. However, all previous studies were reported from single institutions, with no large multi-institutional cohort studies conducted to date.
The 7th Japanese Joint Committee of Lung Cancer Registry (JJCLCR) is one of the most extensive nationwide databases, having collected information on clinicopathological factors from 18,973 patients at 297 hospitals who underwent pulmonary resection for lung cancer in 2010 [11]. Using this database, we reassessed the impact and feasibility of left upper segmentectomy in comparison with left upper lobectomy for c-stage I NSCLC located in the left upper lobe, based on oncological outcomes.
Patients and methods
Ethics statement
The study was approved by the Review Board of Osaka University Hospital (approval no. 15321) following the ethical guidelines for epidemiologic studies, and the need to obtain written informed consent from the patients was waived.
Patients
Patients with c-stage I NSCLC (TNM 8th edition) in the left upper lobe who underwent either left upper lobectomy or segmentectomy in 2010 were enrolled in this study. Although all the data were registered based on the TNM 7th edition, the data regarding c-stage were reviewed based on the TNM 8th edition. The exclusion criteria were as follows: (1) history of thoracic surgery or lung cancer; (2) history of other cancers within five years (3) induction therapy; and (4) combined resection of other adjacent organs, bronchoscopy, or incomplete resection.
Outcomes
Postoperative recurrence was recorded based on the physician’s diagnosis at the institution. The OS was defined as the time from the date of surgery to the date of death from any cause. The recurrence-free survival (RFS) was defined as the time from the date of surgery to the date of local or distant recurrence or death.
Statistical analyses
Descriptive statistics included mean and standard deviation ranges for continuous variables and percentages for categorical variables. The Mann–Whitney U test was used to compare continuous variables, and Fisher’s exact test was used to compare nominal variables. The OS and RFS curves were drawn according to the Kaplan–Meier method, and differences were tested using the log-rank method.
In addition, we conducted a propensity score matching (PSM) analysis to minimize potential data bias using a ratio and caliper distance of 1:1 and 0.05, respectively.
Patients with unreported data were excluded from the analysis due to missing data. Statistical significance was set at p < 0.05.
Results
Oncological outcomes according to the clinical stage in the left upper lobe
A total of 2115 patients who underwent lobectomy or segmentectomy for c-stage I (TNM 8th edition) NSCLC located in the left upper lobe were included in the current study. Among them, 557 patients underwent segmentectomy, and 1558 underwent lobectomy. The demographic and oncological characteristics of patients are shown in Table 1. Age, sex distribution, performance status (PS), smoking history, presence of emphysema and fibrosis, and tumor histology were similar between the groups. Patients who underwent segmentectomy showed a lower pulmonary function, lower serum levels of carcinoembryonic antigen (CEA), lesser extent of lymph node dissection, lower whole and invasive tumor sizes, a better pathological stage, a lower rate of receiving adjuvant therapy, and a lower rate of recurrence than those who underwent lobectomy.
Comparisons between segmentectomy and lobectomy in the left upper lobe after PSM
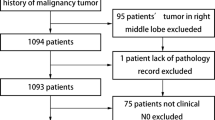
To reduce selection bias, 483 patients who underwent segmentectomy were matched with 483 patients who underwent lobectomy, based on their age, sex, PS, CEA, pulmonary function, and whole and invasive tumor sizes (Fig. 1). The demographic and oncological characteristics of patients post-PSM matching are summarized in Table 2. Even after matching, the patients who underwent segmentectomy exhibited a lesser extent of lymph node dissection, better pathological stage, and a lower rate of receiving adjuvant therapy than those who underwent lobectomy. Overall postoperative complications, including prolonged air leak, did not remarkably vary between the segmentectomy and lobectomy groups (6.2 vs. 8.9%, p = 0.14). In addition, the operative duration was not significantly different between the segmentectomy and lobectomy groups (211 vs. 214 min, p = 0.53).
The median follow-up time was 66.5 ± 0.29 months, calculated using the reverse Kaplan–Meier curve [12]. A comparison of the 5-year RFS and OS rates between segmentectomy and lobectomy cases in each group is shown in Fig. 2. The respective 5-year RFS and OS rates in the segmentectomy and lobectomy groups were 81.9 vs. 78.9% and 87.2 vs. 84.6%, respectively, and the differences between the procedures were not significant (p = 0.15 and p = 0.16, respectively). When stratified by c-stage (UICC ver.8), the respective 5-year RFS and OS rates in each procedure were 84.2 vs. 80.6% (p = 0.082) and 88.3 vs. 85.8% (p = 0.14) in c-stage 0 or IA, and 66.6 vs. 67.2% (p = 0.87) and 79.8 vs. 76.3% (p = 0.80) in stage IB, demonstrating that outcomes between segmentectomy and lobectomy were comparable, irrespective of c-stage. Patients with c-stage IA were divided into c-stage0, c-stage IA1, c-stage IA2, and c-stage IA3, and the 5-year RFS and OS rates were compared for each procedure. The respective 5-year RFS and OS rates in segmentectomy and lobectomy were 95.9 vs. 95.1% (p = 0.82), and 95.9 vs. 97.4% (p = 0.81) in c-stage 0, and 96.1 vs. 86.6% (p = 0.004) and 97.0 vs. 90.5% (p = 0.009) in c-stage IA1, 78.7 vs. 74.6% (p = 0.26) and 85.0 vs. 82.3% (p = 0.52) in stage IA2, 84.4 vs. 89.5% (p = 0.57) and 73.7 vs. 76.9% (p = 0.96) in stage IA3 (Figs. 3 and 4).
The type of recurrence and the incidence of regional and distant recurrences after each procedure are displayed in Table 2. During the follow-up, 56 patients developed recurrence following segmentectomy (11.6%), and 77 developed recurrence following lobectomy (15.9%). Regional recurrence occurred in 35 patients (7.2%) who underwent segmentectomy and 42 patients (8.7%) who underwent lobectomy. Distant metastasis was reported in 25 (5.2%) and 46 (9.3%) patients in the segmentectomy and lobectomy groups, respectively.
Comparison based on radiological findings [ground-glass opacity (GGO)-dominant, solid-dominant, and pure solid tumor] in tumors ≤20 or >20 mm in diameter
Subsequently, we assessed the impact of each procedure on oncological outcomes according to radiological tumor findings. The tumors were classified by the consolidation/tumor ratio (CTR) and divided into 3 groups: GGO-dominant tumor (CTR ≤ 0.5), solid-dominant tumor (CTR > 0.5 to < 1), and pure solid tumor (CTR = 1).
As shown in Fig. 5, in cases where the whole tumor diameter was ≤ 20 mm with GGO- or solid-dominant features, the 5-year RFS and OS rates did not vary markedly between segmentectomy and lobectomy [GGO-dominant: 96.7 vs. 90.4% (p = 0.42) and 96.7 vs. 94.4% (p = 0.17), solid-dominant: 87.4 vs. 89.1% (p = 0.88) and 91.4 vs. 92.6% (p = 0.89)]. Regarding pure solid tumors, segmentectomy showed a better RFS than lobectomy (81.5 vs. 68.5%; p = 0.016). However, the OS was not significantly different between segmentectomy and lobectomy (84.4 vs. 75.6%; p = 0.088).
The aforementioned trend followed the same trend as that of the whole tumor diameter of >20 mm. Even in cases where the whole tumor diameter was >20 mm with GGO- or solid-dominant features, the 5-year RFS and OS rates did not differ markedly between segmentectomy and lobectomy [GGO-dominant; 93.2 vs. 90.5% (p = 0.75) and 98.4 vs. 93.7% (p = 0.44), solid-dominant; 73.3 vs. 69.3% (p = 0.52) and 82.3 vs. 80.3% (p = 0.94)] (Fig. 6). However, for pure solid tumors, the RFS of segmentectomy was worse outcomes than that of lobectomy (57.2 vs. 76.1%; p = 0.028), while the OS was not significantly different between segmentectomy and lobectomy (70.9 vs. 80.3%; p = 0.38). Regarding the type of recurrence for pure solid tumors >20 mm, regional recurrence occurred in 16 patients (18.8%) who underwent segmentectomy and 8 patients 9.8%) who underwent lobectomy (p = 0.092). Distant metastasis was reported in 15 (17.7%) and 14 (17.0%) patients in the segmentectomy and lobectomy groups, respectively (p = 0.92).
Discussion
Recently, multi-institutional randomized clinical trials (JCOG0802/WJOG4607L and CALGB140503) demonstrated the clinical value of segmentectomy in NSCLC. However, this benefit is limited to patients with small-sized peripheral NSCLC [3, 13]. Given the similar anatomical classification of the left upper division and lingula to that of the right upper and middle lobes, those who underwent segmentectomy (i.e. upper division segmentectomy and lingulectomy) in the left upper lobe may benefit in terms of the OS, even for tumors larger than 20 mm in diameter.
Several studies have reported similar oncological outcomes between left upper segmentectomy (upper division segmentectomy and lingulectomy) and left upper lobectomy. Among these studies, one of the largest was conducted by Zhou et al. who performed PSM, resulting in 273 pairs of patients undergoing thoracoscopic left upper division segmentectomy or left upper lobectomy for stage I NSCLC. The authors reported no compelling differences in clinical or oncological outcomes between the groups [9]. Furthermore, our institution also reported that the clinical and oncological outcomes following left upper division segmentectomy for clinical N0 NSCLC were not significantly inferior to those following lobectomy, even if the tumor was located close to the intersegmental plane as measured by three-dimensional computed tomography [10]. Although some studies have demonstrated similar oncological outcomes for left upper segmentectomy and lobectomy (5–9), our study cohort also included patients who underwent other single segmentectomy procedures, such as S1+2 segmentectomy. Since we assumed that all these segmentectomies for the left upper lobe were performed as a ‘split-lobe’ procedure, further studies are required to clarify whether or not the oncological clearance of this split-lobe concept would be applicable for all forms of segmentectomy in the left upper lobe. To our knowledge, this multi-institutional study has the largest cohort showing the non-inferiority of left upper segmentectomy to left upper lobectomy.
Corresponding to previous studies, this study revealed that segmentectomy exhibited an OS comparable to that of left upper lobectomy, irrespective of tumor size. However, the differences between the two groups in terms of pathological characteristics were concerning. Despite adjusting for the characteristics of segmentectomy and lobectomy, including tumor size, the rate of pN1/2 was significantly higher in the lobectomy group than in the segmentectomy group. One reason is that lobe-specific lymph node dissection may have been omitted during segmentectomy. For instance, although complete interlobar lymph node dissection is challenging, owing to variations in the divergence style of the lingular artery and vein during upper division segmentectomy, NSCLC located in the upper division tends to metastasize to the interlobar lymph nodes [14]. As presented in Table 2, mediastinal lymph node dissection during segmentectomy was not performed in some cases, which may have led to underestimation of the pathological stage in the segmentectomy group. Nevertheless, segmentectomy revealed an OS comparable to that of lobectomy. Indeed, a recent study also suggested that the survival was similar between lobectomy and segmentectomy in patients with clinical N0 and unsuspected pathological N1/N2 nodal metastases [15].
One of the most notable concerns after segmentectomy is local recurrence in the residual lobe [16]. Therefore, sufficient tumor margins should be ensured. Theoretically, as the subpleural lymphatic pathway can be blocked by the intersegmental septum, accurate intersegmental dissection during segmentectomy may allow resection of the tumor in the affected segment without the infiltration of cancer cells into the neighboring segment. As margins equal to the tumor diameter or >20 mm would be acceptable [17], and pleural lymphatic drainage might follow an intersegmental pathway [18], dissection into the neighboring segment while sacrificing the intersegmental vein is necessary if the tumor is close to the intersegmental plane. In addition, if the tumor located in the right upper or middle lobe is close to the minor fissure, most thoracic surgeons prefer to spare the middle or upper lobe and dissect into the neighboring lobe to secure the margin instead of performing bi-lobectomy. This may be applicable for large tumors (>20 mm) located in the left upper lobe during split-lobe segmentectomy if the tumor margin is sufficiently secured.
Early-stage NSCLC with a GGO component has been reported to be a uniform group of tumors that exhibit low-grade malignancy and have an excellent prognosis [19]. In contrast, solid predominant or pure solid tumors have more malignant potential, such as lymph node metastasis [20]. Our study revealed that left upper segmentectomy for radiological GGO or solid-dominant stage I NSCLC had long-term effects similar to those of left upper lobectomy, even when the entire tumor size exceeded 20 mm. Interestingly, segmentectomy had a significant advantage with regard to the RFS in patients with solid tumors ≤20 mm in size. One possible reason for this is that pathological upstaging might be infrequent in segmentectomy, probably because segmentectomy may be likely to be applied to cases with peripherally located tumors, which are less strongly associated with lymph node metastasis than inner-located tumor [21]. In contrast, segmentectomy showed a significantly worse RFS than lobectomy for solid tumors >20 mm, as segmentectomy might carry a high risk of recurrence due to insufficient hilar lymph node dissection. Nonetheless, segmentectomy exhibited an OS equivalent to that of lobectomy. One possible reason for this is that segmentectomy, which preserves more of the lung parenchyma than lobectomy, might have allowed more extensive treatment for relapse of primary lung cancer and second primary lung cancer than lobectomy, despite its higher recurrence rate. Thus, segmentectomy offers a prognosis similar to that of lobectomy, even for pure solid tumors.
Several limitations associated with the present study warrant mention. First, despite the propensity matching analysis, selection bias for surgical procedures and other biases may still exist. Indeed, thoracic surgeons performed segmentectomy as curative-intent resection for patients with predominantly GGO, low metabolic activity, slow-growth NSCLC, and compromised limited resection for patients unable to tolerate lobectomy. Second, this dataset, which was based on surgical cases in 2010, lacked information on minimally invasive surgical approaches, such as uniportal-, multiportal-, and robot-assisted thoracoscopic surgery. Third, our dataset lacked information regarding tumor location (i.e. tumor centrality and the specific segment location) and pathological margins. Finally, as the current study was retrospective, further multi-institutional prospective randomized trials are warranted in the future.
In conclusion, the current analyses suggest that the OS following segmentectomy for c-stage I NSCLC in the left upper lobe is not significantly inferior to that following lobectomy, irrespective of the tumor size and radiological tumor findings.
References
Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small-cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–60 (discussion 61).
Tane S, Nishio W, Nishioka Y, Tanaka H, Ogawa H, Kitamura Y, et al. Evaluation of the residual lung function after thoracoscopic segmentectomy compared with lobectomy. Ann Thorac Surg. 2019;108:1543–50.
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–17.
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small-cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22 (discussion 22–3).
Witte B, Wolf M, Hillebrand H, Huertgen M. Split-lobe resections versus lobectomy for lung carcinoma of the left upper lobe: a pair-matched case-control study of clinical and oncological outcomes. Eur J Cardiothorac Surg. 2014;45:1034–9.
Aprile V, Bertoglio P, Dini P, Palmiero G, Mussi A, Ambrogi MC, et al. Is left upper lobectomy always worthwhile for early stage lung cancer? A comparison between left upper lobectomy, trisegmentectomy, and lingulectomy. J Surg Oncol. 2018;117:618–24.
Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg. 2012;144:S23–6.
Iwasaki A, Hamanaka W, Hamada T, Hiratsuka M, Yamamoto S, Shiraishi T, et al. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac Cardiovasc Surg. 2007;55:454–7.
Zhou B, Xu X, Dai J, Guo Y, Jin K, Zhu Y, et al. Propensity matched comparison of VATS left upper tri-segmentectomy and lobectomy. Ann Thorac Surg. 2021;114(3):1007–14.
Nishikubo M, Tane S, Kimura K, Shimizu N, Kitamura Y, Nishio W. Comparison of oncological outcomes between trisegmentectomy and lobectomy for non-small-cell lung cancer in the left upper division. J Thorac Dis. 2022;14:4614–23.
Okami J, Shintani Y, Okumura M, Ito H, Ohtsuka T, Toyooka S, et al. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese Joint Committee of Lung Cancer Registry database in 2010. J Thorac Oncol. 2019;14:212–22.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or Sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388:489–98.
Kuroda H, Sakao Y, Mun M, Uehara H, Nakao M, Matsuura Y, et al. Lymph node metastases and prognosis in left upper division non-small cell lung cancers: the impact of interlobar lymph node metastasis. PLoS ONE. 2015;10: e0134674.
Razi SS, Nguyen D, Villamizar N. Lobectomy does not confer survival advantage over segmentectomy for non-small-cell lung cancer with unsuspected nodal disease. J Thorac Cardiovasc Surg. 2020;159(2469–83): e4.
Nishio W, Yoshimura M, Maniwa Y, Kitamura Y, Tane K, Takenaka D, et al. Re-assessment of intentional extended segmentectomy for clinical T1aN0 non-small cell lung cancer. Ann Thorac Surg. 2016;102:1702–10.
Sawabata N. Locoregional recurrence after pulmonary sublobar resection of non-small-cell lung cancer: can it be reduced by considering cancer cells at the surgical margin? Gen Thorac Cardiovasc Surg. 2013;61:9–16.
Fourdrain A, Epailly J, Blanchard C, Georges O, Meynier J, Berna P. Lymphatic drainage of lung cancer follows an intersegmental pathway within the visceral pleura. Lung Cancer. 2021;154:118–23.
Hattori A, Suzuki K, Takamochi K, Wakabayashi M, Aokage K, Saji H, et al. Prognostic impact of a ground-glass opacity component in clinical stage IA non-small-cell lung cancer. J Thorac Cardiovasc Surg. 2021;161:1469–80.
Inoue M, Minami M, Sawabata N, Utsumi T, Kadota Y, Shigemura N, et al. Clinical outcome of resected solid-type small-sized c-stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg. 2010;37:1445–9.
Decaluwe H, Moons J, Fieuws S, De Wever W, Deroose C, Stanzi A, et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg. 2018;54:134–40.
Acknowledgements
This study was supported by the Japan Lung Cancer Society, Japanese Association for Chest Surgery, Japanese Respiratory Society, Japan Society for Respiratory Endoscopy, and Japanese Association for Thoracic Surgery. The authors thank all institutions that participated in the JJCLCR project. The authors thank Editage (www.editage.com) for the English language editing.
Funding
Open Access funding provided by Kobe University.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tane, S., Okami, J., Maniwa, Y. et al. Clinical outcomes of left upper segmentectomy vs. lobectomy for early non-small-cell lung cancer: a nationwide database study in Japan. Surg Today (2024). https://doi.org/10.1007/s00595-024-02844-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00595-024-02844-8