Abstract
Purpose
The prognostic significance of the cachexia index, a novel biomarker of cancer cachexia, remains unclear in colorectal cancer; we, therefore, evaluated this relationship.
Methods
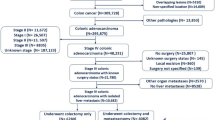
This retrospective cohort study included 306 patients with stage I–III colorectal cancer who underwent R0 resection between April 2010 and March 2020. The cachexia index was calculated as (skeletal muscle index [cm2/m2] × serum albumin level [g/dL])/neutrophil-to-lymphocyte ratio. The overall and disease-free survival rates were analyzed using a Cox proportional hazards model.
Results
A low cachexia index was found in 94 patients. This group had significantly lower disease-free survival and overall survival than the high-cachexia index group (5-year survival, 86.3% vs. 63.1%, p < 0.01; 87.9% vs. 67.2%, p < 0.01). Multivariate analyses showed that T3 or T4 (hazard ratio [HR]: 2.56; 95% confidence interval CI 1.04–6.25, p = 0.039), stage III (HR: 3.77; 95% CI 1.79–7.93, p < 0.01), and a low cachexia index (HR: 2.27; 95% CI 1.31–3.90, p = 0.003) were significant independent predictors of the disease-free survival. CA19-9 ≥ 37.0 ng/mL (HR: 2.68; 95% CI: 1.37–5.24, p = 0.004), stage III (HR: 2.57; 95% CI 1.34–4.92, p = 0.004), and a low cachexia index (HR: 2.35; 95% CI 1.31–4.21, p = 0.004) were significant independent predictors of the overall survival.
Conclusion
A low cachexia index might be a long-term prognostic factor of colorectal cancer.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Kasprzak A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci. 2021;22:1565. https://doi.org/10.3390/ijms22041565.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. https://doi.org/10.1016/S1470-2045(10)70218-7.
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–62. https://doi.org/10.1038/nrc3829.
Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33:90–9. https://doi.org/10.1200/JCO.2014.56.1894.
Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia index in advanced non-small-cell lung cancer patients. Clin Med Insights Oncol. 2015;9:87–93. https://doi.org/10.4137/CMO.S30891.
Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer. 2021;21:563. https://doi.org/10.1186/s12885-021-08300-x.
Go SI, Park MJ, Park S, Kang MH, Kim HG, Kang JH, et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B-cell lymphoma. J Cachexia Sarcopenia Muscle. 2021;12:2211–9. https://doi.org/10.1002/jcsm.12837.
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. https://doi.org/10.1007/s10147-019-01485-z.
Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3rd. English ed. J Anus Rectum Colon 2019 3 175–95
Cremolini C, Antoniotti C, Lonardi S, Bergamo F, Cortesi E, Tomasello G, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer Retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdy140.
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30. https://doi.org/10.1038/sj.bjc.6601242.
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32:1200–5. https://doi.org/10.1016/j.nut.2016.04.003.
Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–7.
Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–7.
Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:3938–46. https://doi.org/10.1245/s10434-014-3815-2.
Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, et al. Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser permanente Northern California population. Cancer Epidemiol Biomarkers Prev. 2017;26:30–7. https://doi.org/10.1158/1055-9965.EPI-16-0145.
Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522–32. https://doi.org/10.1016/j.semcancer.2013.08.007.
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:840. https://doi.org/10.3390/ijms20040840.
Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–9. https://doi.org/10.1038/nature06916.
Pinto I, Carnier N, Oyama J, Otoch JP, Alcântara PS, Tokeshi F, et al. Cancer as a pro-inflammatory environment: metastasis and cachexia. Mediat Inflam. 2015. https://doi.org/10.1155/2015/791060.
Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. BioMed Res Int. 2014. https://doi.org/10.1155/2014/168407.
Cabal-Manzano R, Bhargava P, Torres-Duarte A, Marshall J, Bhargava P, Wainer IW. Proteolysis-inducing factor is expressed in tumours of patients with gastrointestinal cancers and correlates with weight loss. Br J Cancer. 2001;84:1599–601. https://doi.org/10.1054/bjoc.2001.1830.
Schäfer M, Oeing CU, Rohm M, Baysal-Temel E, Lehmann LH, Bauer R, et al. Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol Metab. 2016;5:67–78. https://doi.org/10.1016/j.molmet.2015.11.004.
Toyoshima Y, Kitamura H, Xiang H, Ohno Y, Homma S, Kawamura H, et al. IL6 modulates the immune status of the tumor microenvironment to facilitate metastatic colonization of colorectal cancer cells. Cancer Immunol Res. 2019;7:1944–57. https://doi.org/10.1158/2326-6066.CIR-18-0766.
Wang S, Xie H, Gong Y, Kuang J, Yan L, Ruan G, et al. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep. 2020;10:8153. https://doi.org/10.1038/s41598-020-65091-0.
Abbass T, Tsz Ho YT, Horgan PG, Dolan RD, McMillan DC. The relationship between computed tomography derived skeletal muscle index, psoas muscle index and clinical outcomes in patients with operable colorectal cancer. Clin Nutr ESPEN. 2020;39:104–13. https://doi.org/10.1016/j.clnesp.2020.07.010.
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–31. https://doi.org/10.1016/S1470-2045(15)00558-6.
Crawford J. Clinical results in cachexia therapeutics. Curr Opin Clin Nutr Metab Care. 2016;19:199–204. https://doi.org/10.1097/MCO.0000000000000274.
Takiguchi S, Murakami K, Yanagimoto Y, Takata A, Miyazaki Y, Mori M, et al. Clinical application of ghrelin in the field of surgery. Surg Today. 2015;45:801–7. https://doi.org/10.1007/s00595-014-1040-z.
Naito T, Mitsunaga S, Miura S, Tatematsu N, Inano T, Mouri T, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10:73–83. https://doi.org/10.1002/jcsm.12351.
Hammad A, Kaido T, Uemoto S. Perioperative nutritional therapy in liver transplantation. Surg Today. 2015;45:271–83. https://doi.org/10.1007/s00595-014-0842-3.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. The authors have full control of all primary data and agree to allow the journal to review our data if requested.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the International University of Health and Welfare Hospital (approval no: 22-B-7).
Consent to participate
Written informed consent was obtained from each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamada, T., Haruki, K., Nakashima, K. et al. Prognostic significance of the cachexia index in patients with stage I–III colorectal cancer who underwent laparoscopic surgery. Surg Today 53, 1064–1072 (2023). https://doi.org/10.1007/s00595-023-02646-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02646-4