Abstract
Purpose
Fecal diversion is a less-invasive technique that can alleviate symptoms in patients with refractory anorectal Crohn’s disease. However, complications, including recurrence of residual anorectal Crohn’s disease, may develop. We aimed to evaluate the postoperative results and complications associated with fecal diversion in patients with refractory anorectal Crohn’s disease.
Methods
We enrolled 1218 Crohn’s disease patients who underwent laparotomy at our institute. We retrospectively analyzed the clinical features of 174 patients who underwent fecal diversion for refractory anorectal Crohn’s disease, complications of the diverted colorectum, and the incidence and risk factors for proctectomy after fecal diversion.
Results
After fecal diversion, 74% of patients showed improved symptoms. However, bowel continuity restoration was successful in four patients (2.2%), and anorectal Crohn’s disease recurred in all patients. Seventeen patients developed cancer with a poor prognosis. The rate of conversion to proctectomy after fecal diversion was 41.3%, and the risk factors included rectal involvement (p = 0.02), loop-type stoma (p < 0.01), and the absence of treatment with biologics after fecal diversion (p = 0.03).
Conclusion
Fecal diversion for refractory anorectal Crohn’s disease can improve clinical symptoms. Patients with rectal involvement or loop-type stoma have a greater risk of requiring proctectomy following fecal diversion. The administration of biologic may decrease the rate of proctectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of anorectal lesions in patients with Crohn’s disease (CD) is 20–80% [1,2,3]. A considerable number of such patients (20–49%) undergo surgical intervention, including diverting stoma or proctectomy [4,5,6,7]. Fecal diversion (FD) is less invasive than proctectomy and can alleviate the symptoms; however, many patients experience a worsening of clinical anorectal symptoms after stoma reversal [8]. The European Crohn’s and Colitis Organization (ECCO) stated that pelvic sepsis and its symptoms from complex perianal CD that is refractory to medical or surgical intervention can be controlled through sepsis drainage by a diverting stoma. Additionally, the ECCO stated that a diverting stoma may offer an alternative to extensive resection or proctectomy, allowing time for the acceptance of a permanent stoma due to scant evidence and a decreased rate of fistula healing [9]. However, a meta-analysis reported that the failure of temporary FD requiring proctectomy occurred in 41.6% of cases [8, 10,11,12,13,14,15,16,17,18,19,20]. Additionally, several authors have reported that the independent predictors of proctectomy after fecal diversion include age, the first incidence of anoperineal disease, and rectal involvement by CD [13, 20].
This retrospective single-institution study aimed to evaluate the postoperative results, complications, and rate of proctectomy in patients who undergo FD for refractory or severe anorectal CD.
Materials and methods
Patient selection
We included consecutive CD patients who underwent FD for refractory anorectal lesions between January 1999 and 2017. FD cases for other indications rather than anorectal lesions were not included in the analysis. The characteristics and the clinical course of CD patients were reviewed based on our institutional database and individual chart data.
The data set included sex, the age at the onset of CD, the extent of CD, the type of anorectal lesion (perianal fistula alone, anorectal stricture alone, rectal involvement defined as perirectal abscess or fistula, genital fistula, and perianal fistula with stricture), surgical techniques (i.e., abscess drainage or seton placement), site of stoma, type of initial stoma, duration from the diagnosis of CD to FD, continuous symptoms after FD, and the administration of biologics after FD.
This study was approved by the Ethical Advisory Committee of Yokohama Municipal Citizen's Hospital (21-05-06). The requirement for informed consent was waived because of the retrospective nature of the study.
Perioperative management and surgical procedure
The standard treatments included 5-aminosalicylic acid (3000 mg/day), prednisolone, immuno-modulators (azathioprine or 6-mercaptopurine), and biologics (infliximab [5–10 mg/kg] or adalimumab) for CD-associated lesions without stricture or infection.
FD, which includes the creation of a loop stoma without resection of the diseased colon and rectum or Hartmann’s procedure, is one of the standard surgical procedures for patients with CD with severe colorectal disease. Many Japanese patients tend to select FD to preserve the anus, considering the possibility of subsequent stoma reversal. Younger patients tend to avoid proctectomy with abdominoperineal excision (APE) or total proctocolectomy (TPC) with ileostomy because of the possibility of sexual and urinary dysfunction following these procedures. Proctectomy was defined as APE or TPC. Most loop stomas were constructed in the ileum, except for cases involving patients with a short residual small intestine. Hartmann’s procedure was performed for severe anorectal lesions. However, if the lesions worsened, for example, by the development of continuous purulent discharge from multiple anal fistulae, continuous mucous discharge from the remnant colorectum, or anal pain, proctectomy was performed.
We performed regular follow-up examinations every 2 weeks, for up to 3 months after FD at our outpatient center. The follow-up period was measured as the time from FD to the most recent clinical follow-up examination or death. Follow-up examinations were performed until December 31, 2020.
Outcomes
Outcomes included the incidence of proctectomy in patients with CD after FD, the analysis of uncontrolled Crohn’s anorectal lesions after FD, the restoration of bowel continuity, the cumulative proctectomy rate, and indications for proctectomy (including cancer). Possible risk factors for proctectomy were analyzed to identify significant predictors.
Statistical analysis
Continuous variables were compared using the Mann–Whitney U test. Each factor with a significant P value in a univariate analysis was entered into a stepwise logistic regression model. The data are presented as the median and range. p values of < 0.05 were considered to indicate statistical significance. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for all variables in the univariate analyses. The proctectomy rate after fecal diversion was estimated using the Kaplan–Meier method. All statistical analyses were performed using the R software program (version 4.0.2 2020, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
In total, 174/1218 (14.2%) patients underwent FD because of refractory CD-associated anorectal lesions, defined as actively persistent or symptomatic lesions, wherein optimal medical treatment with surgical drainage had failed. The median observation period from the initial FD was 144 (20–358) months. The baseline characteristics of the 174 patients are presented in Table 1.
All 174 patients underwent open laparotomy during the initial FD without proctectomy. One hundred eighteen of the patients were men, and the median onset age of CD was 19 (range, 4–62) years. In total, 144 patients (82.7%) presented with ileocolic compromise, while 30 patients (17.3%) had colitis. Regarding anorectal lesions (there was some overlap), the indications for FD were as follows: complex perianal fistulae (144 patients), anorectal stricture (109 patients), rectal involvement (58 patients), and genital fistula (39 patients). Ninety-one patients had complicating complex perianal fistula and stricture. In total, 107 patients (61.4%) underwent abscess drainage or seton placement before FD. Regarding the initial site of the stoma, ileostomy and colostomy were performed in 82 (47.1%) and 92 (52.9%) patients, respectively. Regarding the type of initial stoma, loop stoma was performed in 33 patients (18.9%), while the rest underwent Hartmann’s procedure. The median duration from the diagnosis of CD to FD was 142 (range, 4–358) months. Sixty-one (35.0%) patients were treated with biologics after FD. Finally, only four patients (2.2%) underwent stoma reversal. Further, 71 patients underwent proctectomy after FD (71/174, 41.3%). The duration of follow-up after FD when proctectomy was performed was 59 (range, 9–447) months. The follow-up period from FD with and without proctectomy was 136 (20–451) months and 117 (24–323) months, respectively.
Comparison of patients with and without proctectomy after FD
No significant differences in sex, onset of CD, extent of CD, anal lesions (perianal fistula alone, anorectal stricture alone, and genital fistula), stoma site, or duration from the diagnosis of CD to FD were observed between the two groups. The results are summarized in Table 1. However, the patients in the proctectomy group had a significantly higher incidence of rectal involvement and perianal fistula with stricture, higher use of loop stoma/end stoma, longer duration from the diagnosis of CD to FD, and were less frequently treated with biologics after FD, in comparison to the non-proctectomy group (p < 0.01, p = 0.02, p < 0.01, p < 0.01, and p < 0.01, respectively).
Risk factors for proctectomy after FD
Univariate and multivariate analyses were performed to identify independent risk factors for conversion to proctectomy after FD, and the results are presented in Table 2. The multivariate logistic regression analysis identified the following independent risk factors for proctectomy after FD: presence of rectal involvement (HR 1.80; 95% CI 1.13–3.05; p = 0.02), construction of loop-type stoma (HR 2.21; 95% CI 0.36–3.60; p < 0.01), and no administration of biologics after FD (HR 1.92 95% CI 1.05–3.54; p = 0.03) (Table 2).
Analysis of uncontrolled Crohn’s anorectal lesion after FD
Uncontrolled Crohn’s anorectal lesion was defined as unimproved symptoms. In total, 129 (74%) of 174 patients showed improved symptoms. The other 45 patients were classified into the proctectomy and non-proctectomy groups (31 and 14 patients, respectively), and their data were compared. The results are summarized in Table 3. Patients in the proctectomy group had a significantly higher incidence of loop stoma/end than those in the non-proctectomy group (p = 0.01).
Restoration of bowel continuity after FD, cumulative conversion rate, and indications for proctectomy
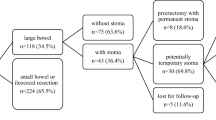
Stoma reversal was performed in 15 of 174 patients (8.6%) (Fig. 1), of which nine patients required re-FD because of an anorectal exacerbation. Four of the nine patients underwent proctectomy, while six underwent anal preservation surgery. Four of the six patients showed restoration of bowel continuity, although they had an anorectal recurrence, while the other two patients who had advanced anorectal cancer died.
The rate of 10-year cumulative proctectomy after FD was 36.7% (Fig. 2). Table 4 shows the indications for proctectomy after FD. The most common indication for proctectomy (47/72 patients) was refractory anorectal disease (65.2%), while the second most common indication was cancer arising from the diverted colon or rectum in 13/72 (18.0%) patients. Complications related to the diverted colon and rectum, including fistulae between the rectum (stump) and functional intestine, active inflammation, and rectal dilatation due to anorectal stricture, were the indications for proctectomy in 10/72 patients (13.8%). Two patients (2.7%) underwent proctectomy for cancer prevention: one had mucus discharge from the residual rectum, and one developed no symptoms after surgery. Overall, there were 17 cancer patients, including two patients with restoration of bowel continuity and two with FD, in whom proctectomy was not possible because of the tumor size and invasion of other organs. Among these patients, 15 died (15/17, 88%) and two survived; however, one patient experienced pelvic recurrence, and the other developed lung metastasis.
Discussion
In this study, we reported that the incidence rate of FD for refractory CD-associated anorectal lesions was 14.2%. A meta-analysis by Singh et al. reported that clinical symptoms improved in two-thirds of patients after FD [8]; similarly, 75% of our patients had an early clinical response. We believe that FD is a relatively effective procedure for treating CD-associated anorectal lesions in terms of symptom relief; however, a few patients may later require proctectomy with a permanent stoma. Therefore, we aimed to determine the proctectomy rate after FD in our center. Several reports have described the proctectomy rates after FD [10,11,12,13,14,15,16,17,18,19,20]. Generally, the proctectomy rate with or without previous FD, ranges from 19.2% to 28.8% [20,21,22,23,24]. The proctectomy rate after FD in our study was 41.3%, which was higher in comparison to previous reports (25%) [13].
In our study, rectal fistula, loop stoma, and non-administration of biologics were identified as independent risk factors for proctectomy after FD. Further, several authors reported that rectal involvement by CD was an independent predictor of proctectomy after FD [13, 20]. These results suggest that rectal involvement may not improve after FD, which was the reason why primary TPC or early completion proctectomy could be considered for this condition [25]. However, no studies have reported loop-type stoma as a risk factor for proctectomy after FD. We speculated that stool flow into the anal side-limb of the loop-type stoma could cause exacerbations of anorectal lesions in our patients. The administration of biologic agents is used to treat patients who do not demonstrate symptomatic improvement after FD. Several studies have reported that biologics were effective for treating perianal disease [20, 26,27,28]; however, they were not effective for reducing the proctectomy rate. Further, regarding FD, biologic agents were not associated with an increased rate of successful restoration of bowel continuity [10, 11, 20, 29]. Our multivariate analysis demonstrated a lower incidence of proctectomy in patients receiving biologics after FD. There are no reports regarding the proctectomy rate of FD before or after the administration of biologics. We speculate that biologics suppress cancer development due to intestinal remission. However, it is necessary to analyze the effects of biologics after FD in a larger population.
Furthermore, regardless of FD, studies have reported that female sex, duration of CD, history of perineal CD, smoking, and the administration of thiopurines are risk factors for proctectomy [22, 24]. We believe that these risk factors require further investigation. The management of uncontrolled Crohn’s anorectal lesions was controversial. In our study, loop stoma was a risk factor for proctectomy. To our knowledge, there are no other reports indicating loop stoma as a risk factor for proctectomy. However, the exact reason could not be clearly stated. Therefore, it is necessary to further examine this issue in a larger population.
Moreover, many reports have discussed stoma reversal after FD [10,11,12,13,14, 20]. In our study, only 15 patients (8.6%) underwent the restoration of bowel continuity, and it was successful in only four patients (2.3%) who ultimately developed recurrence of anorectal lesions. Hain et al. stated that the rate of stoma reversal was 51%, and that the rate of proctectomy was 26% because of the administration of biologics [20]. We did not include the rate of treatment with biologics in this study, and we may introduce biologics actively and consider stoma reversal in future studies. Two patients with restored bowel continuity developed advanced anorectal cancer and died without undergoing surgery. These patients had severe anorectal symptoms, including anal pain and discharge after stoma reversal. Hence, we should pay close attention to the development of cancer in patients with diversion of the colon and rectum, even after performing stoma reversal. In our study, we performed prophylactic proctectomy in two patients. If patients opt for proctectomy, we need to consider it.
The present study was associated with some limitations. First, this was a retrospective study conducted at a single center. Second, the ethnicity of all patients was Japanese, and differences among races and ethnicities in the occurrence of anorectal lesions may exist. In fact, Japanese patients with CD have a higher frequency of anorectal cancer than European or American patients [30]. Third, the criteria employed for the restoration of bowel continuity were ambiguous.
In conclusion, the risk factors for conversion to proctectomy after FD for residual CD-associated anorectal lesions included the presence of rectal involvement, construction of loop-type stoma, and the absence of treatment with biologics. Considering the low rate of restoration of bowel continuity, proctectomy without previous FD may be an alternative in patients with rectal involvement. Similarly, biologics may decrease the proctectomy rate after FD failure.
Data availability
The data underlying this article are available from the corresponding author upon reasonable request.
References
Nordgren S, Fasth S, Hultén L. Anal fistulas in Crohn’s disease: incidence and outcome of surgical treatment. Int J Colorectal Dis. 1992;7:214–8.
Homan WP, Tang C, Thorbjarnarson B. Anal lesions complicating Crohn’s disease. Arch Surg. 1976;111:1333–5.
Fielding JF. Perianal lesions in Crohn’s disease. J R Coll Surg Edinb. 1972;17:32–7.
Galandiuk S, Kimberling J, AI-Mishlab TG, Stromberg AJ. Perianal Crohn disease: predictors of need for permanent diversion. Ann Surg. 2005;5:796–801.
Choi CS, Berg AS, Sangster W, Schieffer KM, Harris LR 3rd, Deiling SM, et al. Combined medical and surgical approach improves healing of septic perianal Crohn’s disease. J Am Coll Surg. 2016;3:506–14.
Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn’s disease. Ann Surg. 2014;260:1057–61.
Mueller MH, Geis M, Glatzel J, Kasparek M, Meile T, Jehle EC, et al. Risk of fecal diversion in complicated perianal Crohn’s disease. J Gastrointest Surg. 2007;11:529–37.
Singh S, Ding NS, Mathis KL, Dulai PS, Farrell AM, Pemberton JH, et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn’ s disease. Aliment Pharmacol Ther. 2015;42:783–92.
Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2020;14:155–68.
Hong MK, Craig Lynch A, Bell S, Woods RJ, Keck JO, Johnston MJ, et al. Faecal diversion in the management of perianal Crohn’s disease. Colorectal Dis. 2011;13:171–6.
Gu J, Valente MA, Remzi FH, Stocchi L. Factors affecting the fate of faecal diversion in patients with perianal Crohn’s disease. Colorectal Dis. 2015;17:66–72.
Harper PH, Kettlewell MG, Lee EC. The effect of split ileostomy on perianal Crohn’s disease. Br J Surg. 1982;69:608–10.
Regimbeau JM, Panis Y, Cazaban L, Pocard M, Bouhnik Y, Matuchansky C, et al. Long-term results of faecal diversion for refractory perianal Crohn’s disease. Colorectal Dis. 2001;3:232–7.
Yamamoto T, Allan RN, Keighley MR. Effect of fecal diversion alone on perianal Crohn’s disease. World J Surg. 2000;24:1258–63.
Edwards CM, George BD, Jewell DP, Warren BF, Mortensen NJ, Kettlewell MG. Role of a defunctioning stoma in the management of large bowel Crohn’s disease. Br J Surg. 2000;87:1063–6.
Grant DR, Cohen Z, McLeod RS. Loop ileostomy for anorectal Crohn’s disease. Can J Surg. 1986;29:32–5.
Orkin BA, Telander RL. The effect of intra-abdominal resection or fecal diversion on perianal disease in pediatric Crohn’s disease. J Pediatr Surg. 1985;20:343–7.
Rehg KL, Sanchez JE, Krieger BR, Marcet JE. Fecal diversion in perirectal fistulizing Crohn’s disease is an underutilized and potentially temporary means of successful treatment. Am Surg. 2009;75:715–8.
Uzzan M, Stefanescu C, Maggiori L, Panis Y, Bouhnik Y, Treton X. Case series: does a combination of anti-TNF antibodies and transient ileal fecal stream diversion in severe Crohn’s colitis with perianal fistula prevent definitive stoma? Am J Gastroenterol. 2013;108:1666–8.
Hain E, Maggiori L, Orville M, Treton X, Bouhnik Y, Panis Y. Diverting stoma for refractory ano-perineal Crohn’s disease: is it really useful in the anti-TNF Era? A multivariate analysis in 74 consecutive patients. J Crohns Colitis. 2019;13:572–7.
Longo WE, Oakley JR, Lavery IC, Church JM, Fazio VW. Outcome of ileorectal anastomosis for Crohn’s colitis. Dis Colon Rectum. 1992;35:1066–71.
O’Riordan JM, O’Connor BI, Huang H, Victor JC, Gryfe R, MacRae HM, et al. Long-term outcome of colectomy and ileorectal anastomosis for Crohn’s colitis. Dis Colon Rectum. 2011;54:1347–534.
Coscia M, Gentilini L, Laureti S, Gionchetti P, Rizzello F, Campieri M, et al. Risk of permanent stoma in extensive Crohn’s colitis. Colorectal Dis. 2013;15:1115–22.
Aaltonen G, Carpelan-Holmström M, Keränen I, Lepistö A. Risk factors for proctectomy in consecutive Crohn’s colitis surgical patients in a reference colorectal centre. Int J Colorectal Dis. 2019;34:1401–6.
Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, et al. ECCO-ESCP consensus on surgery for Crohn’s disease. J Crohns Colitis. 2018;12:1–16.
Present DH, Rutgeets P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fodorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Lichtiger S, Binion DG, Wolf DC, Present DH, Bensimon AG, Wu E, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–39.
Sauk J, Nguyen D, Yajnik V, Khalili H, Konijeti G, Hodin R, et al. Natural history of perianal Crohn’s disease after fecal diversion. Inflamm Bowel Dis. 2014;20:2260–5.
Sasaki H, Ikeuchi H, Bando T, Hirose K, Hirata A, Chohno T, et al. Clinicopathological characteristics of cancer associated with Crohn’s disease. Surg Today. 2017;47:35–41.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for proofreading the manuscript.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Contributions
HK wrote the manuscript. HK, AS, KK, KT, EN, and NO performed the surgeries. HK and AS designed and conducted the research/study, analyzed the data, and interpreted the results. All authors conceived the study, participated in its design and coordination, and helped draft the manuscript. All authors had access to all the data in the study and read and approved the final version of the manuscript. All authors had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuroki, H., Sugita, A., Koganei, K. et al. Postoperative results and complications of fecal diversion for anorectal Crohn’s disease. Surg Today 53, 386–392 (2023). https://doi.org/10.1007/s00595-022-02556-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02556-x