Abstract
Purpose
This retrospective nationwide survey investigated the quality of life (QOL) of patients with esophagogastric junction cancer after gastrectomy using the Postgastrectomy Syndrome Assessment Scale-45.
Methods
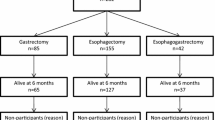
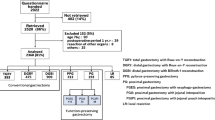
The Postgastrectomy Syndrome Assessment Scale-45 comprises 45 questions classified into symptoms, living status, and QOL domains. A total of 1950 gastrectomized patients with upper-third gastric or esophagogastric junction cancer returned the completed forms. Among them, 224 eligible patients with esophagogastric junction cancer were selected, including 86, 120, and 18 patients who underwent total gastrectomy, proximal gastrectomy (reconstruction-esophagogastrostomy: 56; double-tract method: 51), and other procedures, respectively.
Results
The postoperative period was significantly shorter (47 ± 30 vs. 34 ± 30 months, p = 0.002), and the rates of early-stage disease and minimally invasive approaches significantly higher (both p < 0.001) in the proximal gastrectomy group than in the total gastrectomy group. Despite advantageous background factors for proximal gastrectomy, the postoperative QOL did not differ markedly between the groups. Compared to patients who underwent reconstruction with the double-tract method, patients who underwent esophagogastrostomy had significantly larger remnant stomachs but a similar QOL.
Conclusion
Even with total gastrectomy, a postoperative QOL comparable to that with proximal gastrectomy can be maintained. Clarifying the optimal reconstruction methods for proximal gastrectomy for esophagogastric junction cancer is warranted.
Trial registration
This study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; registration number: 000032221).

Similar content being viewed by others
References
Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7.
Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23(11):1662–5.
Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol. 2011;12(3):296–305.
Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48(11):1624–32.
Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. 2017;20(Suppl 1):69–83.
Rudiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353–61.
Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T, et al. Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg. 2019. https://doi.org/10.2139/ssrn.3321496.
Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7(8):644–51.
Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246(6):992–1000 (discussion 00-1).
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–12.
Japanese Gastric Cancer Association. Japanese Gastric Cancer: A Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01042-y.
Bolton JS, Conway WC 2nd. Postgastrectomy syndromes. Surg Clin North Am. 2011;91(5):1105–22.
Lehnert T, Buhl K. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg. 2004;91(5):528–39.
Katai H. Function-preserving surgery for gastric cancer. Int J Clin Oncol. 2006;11(5):357–66.
Hiki N, Nunobe S, Kubota T, Jiang X. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol. 2013;20(8):2683–92.
Nomura E, Okajima K. Function-preserving gastrectomy for gastric cancer in Japan. World J Gastroenterol. 2016;22(26):5888–95.
Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today. 2016;46(5):517–27.
Nunobe S, Ida S. Current status of proximal gastrectomy for gastric and esophagogastric junctional cancer: a review. Ann Gastroenterol Surg. 2020;4(5):498–504.
Holscher AH, Law S. Esophagogastric junction adenocarcinomas: individualization of resection with special considerations for Siewert type II, and Nishi types EG, E=G and GE cancers. Gastric Cancer. 2020;23(1):3–9.
Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18(1):147–58.
Terashima M, Tanabe K, Yoshida M, Kawahira H, Inada T, Okabe H, et al. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann Surg Oncol. 2014;21(Suppl 3):S370–8.
Namikawa T, Hiki N, Kinami S, Okabe H, Urushihara T, Kawahira H, et al. Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45). Gastric Cancer. 2015;18(2):397–406.
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18(2):407–16.
Kawahira H, Kodera Y, Hiki N, Takahashi M, Itoh S, Mitsumori N, et al. Optimal Roux-en-Y reconstruction after distal gastrectomy for early gastric cancer as assessed using the newly developed PGSAS-45 scale. Surg Today. 2015;45(10):1307–16.
Inada T, Yoshida M, Ikeda M, Yumiba T, Matsumoto H, Takagane A, et al. Evaluation of QOL after proximal gastrectomy using a newly developed assessment scale (PGSAS-45). World J Surg. 2014;38(12):3152–62.
Misawa K, Terashima M, Uenosono Y, Ota S, Hata H, Noro H, et al. Evaluation of postgastrectomy symptoms after distal gastrectomy with Billroth-I reconstruction using the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45). Gastric Cancer. 2015;18(3):675–81.
Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, et al. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2015;19(1):302–11.
Tanizawa Y, Tanabe K, Kawahira H, Fujita J, Takiguchi N, Takahashi M, et al. Specific features of dumping syndrome after various types of gastrectomy as assessed by a newly developed integrated questionnaire, the PGSAS-45. Dig Surg. 2016;33(2):94–103.
Nakada K, Takahashi M, Ikeda M, Kinami S, Yoshida M, Uenosono Y, et al. Factors affecting the quality of life of patients after gastrectomy as assessed using the newly developed PGSAS-45 scale: A nationwide multi-institutional study. World J Gastroenterol. 2016;22(40):8978–90.
Takahashi M, Terashima M, Kawahira H, Nagai E, Uenosono Y, Kinami S, et al. Quality of life after total vs distal gastrectomy with Roux-en-Y reconstruction: use of the postgastrectomy syndrome assessment scale-45. World J Gastroenterol. 2017;23(11):2068–76.
Tanabe K, Takahashi M, Urushihara T, Nakamura Y, Yamada M, Lee SW, et al. Predictive factors for body weight loss and its impact on quality of life following gastrectomy. World J Gastroenterol. 2017;23(26):4823–30.
Kinami S, Takahashi M, Urushihara T, Ikeda M, Yoshida M, Uenosono Y, et al. Background factors influencing postgastrectomy syndromes after various types of gastrectomy. World J Clin Cases. 2018;6(16):1111–20.
Yabusaki H, Kodera Y, Fukushima N, Hiki N, Kinami S, Yoshida M, et al. Comparison of postoperative quality of life among three different reconstruction methods after proximal gastrectomy: insights from the PGSAS study. World J Surg. 2020;44(10):3433–40.
Turner-Bowker DM, Bayliss MS, Ware JE Jr, Kosinski M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res. 2003;12(8):1003–12.
Svedlund J, Sjodin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–34.
Kulich KR, Madisch A, Pacini F, Pique JM, Regula J, Van Rensburg CJ, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes. 2008;6:12.
Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35(2):357–64.
Shan B, Shan L, Morris D, Golani S, Saxena A. Systematic review on quality of life outcomes after gastrectomy for gastric carcinoma. J Gastrointest Oncol. 2015;6(5):544–60.
van den Boorn HG, Stroes CI, Zwinderman AH, Eshuis WJ, Hulshof M, van Etten-Jamaludin FS, et al. Health-related quality of life in curatively-treated patients with esophageal or gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;154:103069.
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2016;265(2):277–83.
Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010;97(4):558–62.
Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer. 2019;22(5):1029–35.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60.
Takiguchi S, Murakami K, Yanagimoto Y, Takata A, Miyazaki Y, Mori M, et al. Clinical application of ghrelin in the field of surgery. Surg Today. 2015;45(7):801–7.
Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, et al. Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology. 2012;59(114):633–40.
Tanioka T, Waratchanont R, Fukuyo R, Saito T, Umebayashi Y, Kanemoto E, et al. Surgical and nutritional outcomes of laparoscopic proximal gastrectomy versus total gastrectomy: a meta-analysis. Surg Endosc. 2020;34(3):1061–9.
Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg. 2013;37(3):558–64.
Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, et al. Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer. 2014;17(1):141–5.
Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29(12):1592–9.
Nomura E, Lee SW, Tokuhara T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and type of reconstruction following open and laparoscopic proximal gastrectomy for gastric cancer. Hepatogastroenterology. 2012;59(118):1677–81.
Park DJ, Kim HH, Han SU, Hyung WJ, Hwang SH, Hur H, et al. Multicenter prospective randomized controlled trial of comparing laparoscopic proximal gastrectomy and laparoscopic total gastrectomy for upper third early gastric cancer (KLASS-05). J Clin Oncol. 2019;37(4):TPS184.
Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, et al. Double-flap technique as an antireflux procedure in esophagogastrostomy after proximal gastrectomy. J Am Coll Surg. 2016;223(2):e7–13.
Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol. 2017;24(6):1635–42.
Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A, et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann Gastroenterol Surg. 2019;3(1):96–103.
Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20(4):728–35.
Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg. 2013;398(5):697–701.
Acknowledgements
The authors thank all physicians who participated in this study and the patients whose cooperation made this study possible.
Funding
This study was supported by grants from the Jikei University and the Japanese Gastric Cancer Association. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, SW., Kaji, M., Uenosono, Y. et al. The evaluation of the postoperative quality of life in patients undergoing radical gastrectomy for esophagogastric junction cancer using the Postgastrectomy Syndrome Assessment Scale-45: a nationwide multi-institutional study. Surg Today 52, 832–843 (2022). https://doi.org/10.1007/s00595-021-02400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02400-8