Abstract
Small-for-size graft (SFSG) syndrome after living donor liver transplantation (LDLT) is the dysfunction of a small graft, characterized by coagulopathy, cholestasis, ascites, and encephalopathy. It is a serious complication of LDLT and usually triggered by excessive portal flow transmitted to the allograft in the postperfusion setting, resulting in sinusoidal congestion and hemorrhage. Portal overflow injures the liver directly through nutrient excess, endothelial activation, and sinusoidal shear stress, and indirectly through arterial vasoconstriction. These conditions may be attenuated with portal flow modulation. Attempts have been made to control excessive portal flow to the SFSG, including simultaneous splenectomy, splenic artery ligation, hemi-portocaval shunt, and pharmacological manipulation, with positive outcomes. Currently, a donor liver is considered a SFSG when the graft-to-recipient weight ratio is less than 0.8 or the ratio of the graft volume to the standard liver volume is less than 40%. A strategy for transplanting SFSG safely into recipients and avoiding extensive surgery in the living donor could effectively address the donor shortage. We review the literature and assess our current knowledge of and strategies for portal flow modulation in LDLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to severe organ shortages and the increasing gap between supply and demand, living donor liver transplantation (LDLT) has become an accepted alternative to expand the donor pool [1,2,3,4]. Variables such as the model for the end-stage liver disease score, donor/recipient age, recipient body mass index, and pretransplant diagnosis reportedly allow for the prediction of short-term mortality after liver transplantation [5,6,7]. However, the use of partial hepatic grafts in LDLT can complicate the prediction [8]. Since the introduction of adult LDLT, graft size has become a concern, particularly for patients with Child class C cirrhosis and/or portal hypertension. Small-for-size graft (SFSG) syndrome after LDLT is a major complication of this procedure [9,10,11,12]. It is defined as the dysfunction of a small graft within the first 1–2 post-transplant weeks in the absence of any other identifiable cause and is characterized by coagulopathy, cholestasis, ascites, and encephalopathy [13, 14]. A key mechanism of SFSG syndrome is thought to be excessive portal flow transmitted to the allograft in the postperfusion setting, resulting in sinusoidal congestion and hemorrhage [13]. In the setting of portal overflow, adaptive responses in the liver lead to vasoconstriction of the hepatic artery [15]. Whereas portal overflow injures the liver directly through nutrient excess, endothelial activation, and sinusoidal shear stress, arterial vasoconstriction introduces secondary ischemic damage [16, 17]. These insults may be attenuated with portal flow modulation. Attenuating portal flow increases arterial flow and improves graft function [18, 19].
Currently, a donor liver is considered a SFSG when the graft-to-recipient weight ratio (GRWR) is less than 0.8% or the ratio of the graft volume (GV) to the standard liver volume (SLV) is less than 40% [18, 20]. Factors other than the GV can potentially influence outcomes. These factors include recipient-related factors such as clinical disease status and portal hypertension; graft-related factors such as donor age, steatosis, and immunological factors, and technical factors such as vascular reconstruction, adequate hepatic venous outflow, and vascular inflow [8, 20,21,22,23]. For example, grafts within a wide range of GV-to-SLV ratios can tolerate portal hypertension if they have excellent venous outflow capacity, but when the ratio range is exceeded, modulation of vascular inflow becomes necessary for graft survival [19].
One Korean group no longer measures portal pressures routinely. Rather, they consider establishing excellent venous outflow, preventing acute rejection, and having young donors to be key mechanisms for avoiding SFSG syndrome in grafts with a GRWR as small as 0.7%. That group noticed that successful SFSGs were from young donors with young recipients in whom perfect venous outflow was ensured [24]. They maintain that portal venous pressure (PVP) > 20 mmHg is not associated with an increased risk of SFSG syndrome or graft loss, as long as perfect venous outflow is maintained and portal flow steal is interrupted with portosystemic shunt ligation [13, 24].
It is important to consider donor safety and avoid subjecting healthy living donors to excessive surgery [25,26,27,28]. A strategy for transplanting SFSG safely into recipients while avoiding excessive surgery in living donors could effectively address the donor shortage [20]. Several strategies to prevent SFSG syndrome, including portal flow and/or hepatic venous outflow modulation, have been reported [4, 9, 29]. Excessive portal flow and/or the reduced intrahepatic vascular bed result in higher portal flow, increased portal pressure, and stress at the hepatic sinusoid [30, 31]. To reduce the risk of these factors, different portal flow modulation techniques have been described. We review the relevant literature and assess the current knowledge of and strategies for portal flow modulation in LDLT.
Splenectomy
Generally, splenectomy is performed to reduce the bleeding tendency resulting from thrombocytopenia, or as part of surgical procedures such as devascularization of the upper stomach and esophageal transection to control variceal hemorrhage. It is also performed for hematologic disease such as idiopathic thrombocytopenic purpura, splenic tumors such as malignant lymphoma, trauma, splenic artery aneurysm, and liver or renal transplantation from an ABO-incompatible donor [32]. In rodent models, splenectomy improves the vascular compliance of the graft and increases hepatic serotonin, which plays a significant role in hepatic perfusion through its vasodilatory effects [33, 34]. Hepatic serotonin improves microcirculation and promotes liver regeneration by stimulating the endothelial cells to release vascular endothelial growth factor. It also protects the graft by increasing the microcirculation and accelerating liver regeneration [35].
Simultaneous splenectomy during LDLT improves graft outcomes by reducing the portal pressure and flow and increasing the vascular compliance of the graft [36,37,38]. We previously reported that simultaneous splenectomy reduced hypersplenism and prevented graft congestion resulting from excessive portal flow [9, 39]. In our first report, the outcomes of six cases of LDLT with a left-lobe graft were analyzed. None of the patients who underwent splenectomy suffered hyperbilirubinemia or intractable ascites. Both portal pressure and portal vein flow decreased after splenectomy in most of the patients. To clarify whether splenectomy was beneficial for patients with a SFSG, further analysis was performed for patients who had a GV-to-SLV ratio of 40% or lower (n = 50) [9]. SFSG syndrome developed in 11 of 50 patients with a GV-to-SLV ratio of 40% or lower, and excluding splenectomy was an independent risk factor for SFSG syndrome in our patients. Kaido et al. clarified that overall survival rates after LDLT were significantly higher for patients with a final PVP ≤ 15 mmHg than for those with a PVP > 15 mmHg [40]. Therefore, they routinely apply a portal pressure control program that targets a final PVP ≤ 15 mmHg to prevent SFSG syndrome. The Kyoto group recently reported re-evaluating the indications for PVP modulation, which they achieve primarily with splenectomy [41]. They found that failed PVP modulation (final PVP > 15 mmHg) was associated with an increased incidence of SFSG syndrome and early graft loss. Among 38 patients with failed PVP modulation, donor age ≥ 45 years and ABO incompatibility were independent risk factors for graft loss [41]. Survival analysis showed that PVP > 15 mmHg was related to poor prognosis in grafts from either ABO-incompatible donors or from donors ≥ 45 years of age, but it did not negatively affect grafts from ABO-compatible/identical donors or from donors < 45 years of age. They concluded that PVP modulation is not necessary in all recipients. Grafts from ABO-compatible/identical donors and from donors < 45 years of age can tolerate portal hypertension; however, lowering the final PVP to ≤ 15 mmHg is necessary for patients with grafts from ABO-incompatible donors or from donors ≥ 45 years of age [41]. In most reports, the decision to perform splenectomy was made after graft implantation. Portal hyperperfusion can injure the SFSG when splenic flow is present. Therefore, we perform splenectomy before graft implantation to prevent graft injury if portal hypertension is anticipated, because of splenomegaly and excessive portal flow. Among 258 patients who underwent splenectomy for portal flow modulation during LDLT at Kyushu University, 232 (89.9%) underwent splenectomy before implantation. In a rodent SFSG liver transplant model, better outcomes were achieved when splenectomy was performed just before partial liver transplantation (LT) than after partial LT because of the direct elimination of splenic inflammatory leukocytes and inhibition of inflammatory leukocyte infiltration [42].
In Japan, the benefits of simultaneous splenectomy in LDLT are well documented, whereas the negative effects, including potential adverse events such as portal venous thrombosis (PVT), infectious complications, pancreatic fistula, and postoperative bleeding, are not discussed in detail [43]. Ito et al. recently reported a significantly higher incidence of reoperation for postoperative hemorrhage within the first postoperative week, and of lethal infectious disease as well as greater intraoperative blood loss and longer surgery time among recipients who undergo simultaneous splenectomy than among those who do not [43]. Selected patients in that series underwent splenectomy at the time of LDLT if medically indicated, but never for PVP modulation. Patients who underwent splenectomy did not have a lower incidence of SFSG syndrome than those who did not. Notably, a GV-to-SLV ratio above 40% was required and the donors [43] were younger than those in the Kyoto study (median, 35 years old vs. 45 years old, respectively) [41]. A vessel sealing system for dissection around the spleen and a vascular stapler for the splenic hilum have improved the ease and safety of splenectomy, even for patients with severe portal hypertension [9, 44]. Furthermore, the splenic artery is routinely ligated when simultaneous splenectomy is performed. Consequently, intraoperative blood loss and surgery time are not increased when simultaneous splenectomy is performed at our center. However, more technical refinement is necessary to prevent pancreatic fistula and bleeding from the splenic stump.
Portal vein thrombosis after splenectomy
PVT is a severe complication of LDLT that can result in increased morbidity and mortality [45]. The incidence of PVT in deceased donor LT ranges from 0.3 to 2.6% [46, 47], and increases to 4–9% in adult LDLT with more complex surgical techniques and complicated vascular reconstructions, mainly related to shorter vessel grafts resulting in shorter vessel length for anastomosis [48, 49]. PVT is not a rare complication of splenectomy for patients with cirrhosis in a non-transplant setting [32, 50]. In patients with cirrhosis, decreased portal flow and the development of portosystemic collaterals are considered predisposing factors for PVT. Furthermore, the imbalance between coagulation factors and coagulation inhibitory factors resulting from decreased levels of coagulation inhibitory factors such as protein C, protein S, and antithrombin-III, may cause PVT in these patients [32, 50]. Kinjo et al. also reported that the incidence of PVT after splenectomy in a non-transplant setting was 24.3%. The independent risk factors for PVT in that study were large splenic vein diameter (13 mm or more) and low white cell count (≤ 2 × 103 /mm3), and spleen weight was correlated with splenic vein diameter and white cell count [51].
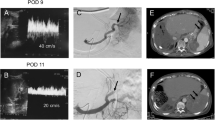
The relationship between splenectomy and an increased incidence of PVT after LDLT is not clear. Kurata et al. reported a very high incidence of PVT (33.3%) after LDLT with splenectomy, but no incidence after LDLT without splenectomy [52]. They concluded that using grafts of sufficient size was the key to controlling PVP and that splenectomy, a risk factor for PVT, should be avoided whenever possible in LDLT [52]. Blood stasis in the stump of the splenic vein results in thrombosis, which subsequently extends to the portal and superior mesenteric veins [32, 51, 53]. Figure 1 shows a PVT extending from the splenic vein stump after LDLT with simultaneous splenectomy. This patient underwent emergency thrombectomy, which was effective and there was no recurrence of PVT. Anticoagulant therapy was not given to this patient. The Kyoto group reported that the incidence of PVT did not differ between patients with splenectomy (5.7%) and without (2.6%), whereas the incidence of isolated splenic vein thrombosis was higher in patients with splenectomy (6.8%) and required short-term anticoagulant therapy [53]. Although patients with isolated splenic vein thrombosis are not given anticoagulants at Kyushu University, the incidence of PVT after LDLT is not higher at this institution than in the Kyoto group (Fig. 2). Further study is needed to establish whether anticoagulant therapy is recommended for patients with splenic vein thrombosis after splenectomy.
Incidence of portal vein thrombosis (PVT). The incidence of PVT after LDLT was 3.5% with simultaneous splenectomy and 4.0% with splenectomy before LDLT. In contrast, no PVT was detected in patients without portal flow modulation or in those who underwent SAL. The difference among the groups was not significant
Splenic artery ligation
Splenic artery ligation (SAL) was initially applied to prevent thrombocytopenia in LDLT [54]. Troisi et al. proposed the use of SAL to resolve ascites and increase hepatic arterial flow in LDLT [55]. Moreover, they reported that SAL was a simple and effective method for decreasing portal flow when recipient portal venous flow did not exceed 500 ml/min per 100 g of liver. Other techniques, such as portocaval shunt or portomesenteric disconnection should be considered when SAL is insufficient to relieve portal hypertension [56]. Therefore, SAL is an alternative to splenectomy for reducing PVP and flow [57]. Ishizaki et al. reported their successful experience of LDLT using left-lobe grafts without portal flow modulation [58]. In their study, the mean GRWR was 0.82% and 6 of the 42 patients underwent SAL. At Kyushu University Hospital, recipients who underwent SAL had worse graft function after LDLT than those who underwent splenectomy [9, 39]. Moreover, the 1-year graft survival rate was 91.2% for recipients with splenectomy, but only 77.9% for those with SAL in our single-center experience. This suggests that SAL was insufficient to modulate excessive portal flow and/or pressure compared with splenectomy. Umeda et al. established the preoperative proximal splenic artery embolization technique to prevent intraoperative bleeding resulting from injury to the massive collateral vessels around the splenic artery [59]. They embolized the proximal splenic artery with interventional radiology 12–18 h before LDLT without complications such as sepsis, portal thrombus, or abscess formation. They concluded that splenic artery embolization reduced excessive portal flow and improved graft function, which lowered the incidence of SFSG syndrome and had advantages for liver regeneration [60]. Since patients who wait for LDLT often have thrombocytopenia and coagulopathy, pseudoaneurysm of the punctured artery or bleeding around the puncture site should be considered and checked when performing this technique. Furthermore, this technique has a substantial risk of pancreatitis and postembolization syndrome [61]. Moon et al. recently reported the effects of a splenic devascularization procedure to prevent various complications, including PVT, pancreatic fistula, and bleeding from the splenic stump after splenectomy [61]. They performed not only SAL but also ligation of the right gastroepiploic artery and division of the gastrosplenic ligament including the short gastric arteries as an alternative to splenectomy. Since arterial supply to the spleen is maintained by intrapancreatic collaterals from the superior mesenteric artery, there was no incidence of splenic infarction and/or abscess. The splenic volume decreased to 60% of the original volume for 1 month after LDLT with splenic devascularization. Although the incidence of SFSG syndrome was similar in the two groups, procedure-related complications were less common in patients who underwent devascularization than in those who underwent splenectomy. The mean GRWR at their institution was 1.1% and splenectomy or devascularization was performed in only 10.6% of patients. Further studies from other institutions that use smaller grafts would clarify the impact of this procedure.
Portocaval shunt
Compensatory portosystemic shunts develop in about 40% of patients with cirrhosis and the frequency increases with severity [62, 63]. Large shunts can be an advantage during recipient hepatectomy because the effect of severe portal hypertension is significantly reduced and portal clamping results in less congestion in the mesenteric system [63, 64]. Spontaneous portosystemic shunts are commonly removed during standard LT because the escape of portal inflow through collaterals (steal phenomenon) may lead to ischemic graft damage [65] and PVT (Fig. 3). Troisi et al. recommended that spontaneous portosystemic shunts be left in place when small grafts (GRWR < 0.8%) with hyperkinetic portal flow are used in LDLT [56]. The Kyoto group reported that the ligation of large portosystemic shunts increases portal venous flow and to prevent the portal venous steal phenomenon after LDLT when graft resistance increases during rejection [40]. The Asan group reported surgical interruption of portosystemic collaterals and additional evaluation of collaterals with intraoperative cine-portogram in recipients with large portosystemic shunts, which are a possible route of postoperative portal flow steal [61, 66, 67]. We ligate large shunts to maintain adequate portal flow and to prevent the steal phenomenon as long as the portal pressure does not exceed 20 mmHg after test clamping [8, 21, 68]. Balloon-occluded retrograde transvenous obliteration is a feasible intervention to close the remnant shunt when portal flow decreases because of the steal phenomenon [69]. Takatsuki et al. opposed mandatory ligation of shunts because 75% of their patients experienced no complications without ligation and the shunt ligation procedure can be dangerous [70].
Representative case of a patient with a huge portosystemic shunt. a, b Pre-LDLT: enhanced CT shows huge splenorenal (arrow head) and mesocaval (arrowhead) shunts. The portal vein was atrophic (arrow). c, d 3 months after LDLT with splenectomy. Enhanced CT reveals a patent mesocaval shunt (arrowhead). The portal vein (arrow) was thrombosed. The splenorenal shunt was closed with simultaneous splenectomy
To prevent graft failure resulting from portal hyperperfusion, diversion of the superior mesenteric flow with a mesocaval shunt was first described for LDLT using a SFSG [71]. Animal experiments using a portocaval shunt in SFSG transplant have demonstrated that adequate decompression of the portal system can effectively prevent the sinusoidal congestion and graft injury typically seen in SFSG syndrome [72, 73]. A hemi-portocaval shunt reduced portal flow and improved patient and graft survival by preventing SFSG syndrome in the clinical setting [74-76]. It is important to rule out hepatic venous outflow obstruction before creating a shunt because a shunt in the presence of this obstruction can cause severe portal steal and graft ischemia [63]. Hemi-portocaval shunts may result in excessive diversion of the portal flow into the systemic circulation and may lead to the steal phenomenon. Troisi et al. recommended measuring portal flow and calibrating the size of the shunt accordingly [75]. It remains controversial whether these shunts should be closed after graft regeneration occurs and liver function stabilizes [76, 77].
Pharmacologic manipulation
Pharmacologic manipulation is another method of portal flow modulation. This method is reversible, unlike surgical procedures such as splenectomy or SAL. Propranolol and somatostatin decrease both portal flow and portal pressure reliably and reproducibly in cirrhotic patients [78]; therefore, these drugs are used widely in the treatment of variceal bleeding [79]. A couple of studies reported the effect of somatostatin infusion in an animal model of LT with SFSG [80, 81]. Xu et al. found that somatostatin improved 7-day graft survival by attenuating acute-phase shear stress. Hessheimer et al. revealed that somatostatin reduced portal vein flow and protected sinusoidal endothelial cells, and that somatostatin had a cytoprotective effect on hepatic stellate cells [81]. In a recent randomized trial, Troisi et al. found that somatostatin decreased the hepatic venous portal gradient and preserved arterial flow to the graft [82]. The beneficial effects of the intraportal infusion of prostaglandin E1 (PGE1), a vasodilator with hepatoprotective effects, have been reported [83, 84]. We previously reported that the continuous intraportal infusion of PGE1, nafamostat mesylate, and a thromboxane synthetase inhibitor prevented SFSG syndrome after LDLT by attenuating microcirculatory insufficiency [85]. Moreover, the continuous intraportal infusion of PGE1 was effective in split LT for two adults [86]. Microthrombus in the portal vein, caused by inserting an indwelling catheter when performing continuous intraportal infusion therapy, should be avoided.
In conclusion, portal flow modulation with a prudent combination of shunt control such as ligation or hemi-portocaval shunt, a portal decompression procedure such as simultaneous splenectomy or SAL, and pharmacologic manipulation, is crucial when performing LDLT with an SFSG.
Conflict of interest
Tomoharu Yoshizumi and Masaki Mori have no conflicts of interest to declare.
Change history
08 January 2020
The article Portal flow modulation in living donor liver transplantation: review
08 January 2020
The article Portal flow modulation in living donor liver transplantation: review
Abbreviations
- LDLT:
-
Living donor liver transplantation
- SFSG:
-
Small-for-size graft
- GRWR:
-
Graft-to-recipient weight ratio
- GV:
-
Graft volume
- SLV:
-
Standard liver volume
- LT:
-
Liver transplantation
- PVT:
-
Portal vein thrombosis
- SAL:
-
Splenic artery ligation
- HVOO:
-
Hepatic venous outflow obstruction
- PGE1:
-
Prostaglandin E1
References
Makuuchi M. Living donor liver transplantation: looking back at my 30 years of experience. Surg Today. 2019;49:288–94.
Imai D, Yoshizumi T, Sakata K, Ikegami T, Itoh S, Harada N, et al. Long-term outcomes and risk factors after adult living donor liver transplantation. Transplantation. 2018;102:e382–e391391.
Yoshizumi T, Takada Y, Shirabe K, Kaido T, Hidaka M, Honda M, et al. Impact of human T-cell leukemia virus type 1 on living donor liver transplantation: a multi-center study in Japan. J Hepatobiliary Pancreat Sci. 2016;23:333–41.
Goldaracena N, Echeverri J, Selzner M. Small-for-size syndrome in live donor liver transplantation-pathways of injury and therapeutic strategies. Clin Transplant. 2017;31:e12885.
Alexopoulos S, Matsuoka L, Cho Y, Thomas E, Sheikh M, Stapfer M, et al. Outcomes after liver transplantation in patients achieving a model for end-stage liver disease score of 40 or higher. Transplantation. 2013;95:507–12.
Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol. 2012;57:1135–40.
Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–26.
Yoshizumi T, Taketomi A, Uchiyama H, Harada N, Kayashima H, Yamashita Y, et al. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007–133.
Yoshizumi T, Taketomi A, Soejima Y, Ikegami T, Uchiyama H, Kayashima H, et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transpl Int. 2008;21:833–42.
Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–10.
Taniguchi M, Shimamura T, Todo S, Furukawa H. Small-for-size syndrome in living-donor liver transplantation using a left lobe graft. Surg Today. 2015;45:663–71.
Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, et al. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581–6.
Etesami K, Genyk Y. The increasingly limited basis for portal venous pressure modulation in living donor liver transplantation. Liver Transpl. 2018;24:1506–7.
Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, et al. Feasibility of left lobe living donor liver transplantation between adults: an 8-year, single-center experience of 107 cases. Am J Transplant. 2006;6:1004–111.
Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. 1990;98:1024–8.
Cantre D, Schuett H, Hildebrandt A, Dold S, Menger MD, Vollmar B, et al. Nitric oxide reduces organ injury and enhances regeneration of reduced-size livers by increasing hepatic arterial flow. Br J Surg. 2008;95:785–92.
Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–9.
Emond JC, Goodrich NP, Pomposelli JJ, Baker TB, Humar A, Grant DR, et al. Hepatic hemodynamics and portal flow modulation: the A2ALL experience. Transplantation. 2017;101:2375–84.
Chan SC, Lo CM, Chok KS, Sharr WW, Cheung TT, Tsang SH, et al. Modulation of graft vascular inflow guided by flowmetry and manometry in liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:649–56.
Troisi RI, Sainz-Barriga M. Successful transplantation of small-for-size grafts: a reappraisal. Liver Transpl. 2012;18:270–3.
Yoshizumi T, Ikegami T, Bekki Y, Ninomiya M, Uchiyama H, Iguchi T, et al. Re-evaluation of the predictive score for 6-month graft survival in living donor liver transplantation in the modern era. Liver Transpl. 2014;20:323–32.
Yoshizumi T, Taketomi A, Soejima Y, Uchiyama H, Ikegami T, Harada N, et al. Impact of donor age and recipient status on left-lobe graft for living donor adult liver transplantation. Transpl Int. 2008;21:81–8.
Toshima T, Ikegami T, Matsumoto Y, Yoshiya S, Harimoto N, Yamashita Y, et al. One-step venous reconstruction using the donor’s round ligament in right-lobe living-donor liver transplantation. Surg Today. 2015;45:522–5.
Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17–38.
Shin M, Song S, Kim JM, Kwon CH, Kim SJ, Lee SK, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation. 2012;93:942–8.
Reichman TW, Sandroussi C, Azouz SM, Adcock L, Cattral MS, McGilvray ID, et al. Living donor hepatectomy: the importance of the residual liver volume. Liver Transpl. 2011;17:1404–11.
Dayangac M, Taner CB, Yaprak O, Demirbas T, Balci D, Duran C, et al. Utilization of elderly donors in living donor liver transplantation: when more is less? Liver Transpl. 2011;17:548–55.
Yoshizumi T, Ikegami T, Kimura K, Uchiyama H, Ikeda T, Shirabe K, et al. Selection of a right posterior sector graft for living donor liver transplantation. Liver Transpl. 2014;20:1089–96.
Toshima T, Yoshizumi T, Shimokawa M, Ikegami T, Harada N, Itoh S, et al. Feasibility of all-in-one venoplasty with a venous cuff using an opened round ligament for the right lobe graft in living donor liver transplantation. Liver Transpl. 2019;25:171–5.
Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl. 2003;9:S36–41.
Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986–93.
Kawanaka H, Akahoshi T, Kinjo N, Konishi K, Yoshida D, Anegawa G, et al. Impact of antithrombin III concentrates on portal vein thrombosis after splenectomy in patients with liver cirrhosis and hypersplenism. Ann Surg. 2010;251:76–83.
Nagao Y, Akahoshi T, Kamori M, Uehara H, Hashimoto N, Kinjo N, et al. Liver regeneration is promoted by increasing serotonin content in rat liver with secondary biliary cirrhosis. Hepatol Res. 2011;41:784–94.
Tian Y, Graf R, El-Badry AM, Lesurtel M, Furrer K, Moritz W, et al. Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology. 2011;53:253–62.
Yoshizumi T, Itoh S, Imai D, Ikegami T, Ninomiya M, Iguchi T, et al. Impact of platelets and serotonin on liver regeneration after living donor hepatectomy. Transplant Proc. 2015;47:683–5.
Athanasiou A, Moris D, Damaskos C, Spartalis E. Splenectomy is not indicated in living donor liver transplantation. Liver Transpl. 2017;23:561–2.
Wang H, Ikegami T, Harada N, Yoshizumi T, Soejima Y, Uchiyama H, et al. Optimal changes in portal hemodynamics induced by splenectomy during living donor liver transplantation. Surg Today. 2015;45:979–85.
Ogura Y, Hori T, El Moghazy WM, Yoshizawa A, Oike F, Mori A, et al. Portal pressure < 15 mm Hg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl. 2010;16:718–28.
Shimada M, Ijichi H, Yonemura Y, Harada N, Shiotani S, Ninomiya M, et al. The impact of splenectomy or splenic artery ligation on the outcome of a living donor adult liver transplantation using a left lobe graft. Hepatogastroenterology. 2004;51:625–9.
Kaido T, Ogawa K, Fujimoto Y, Ito T, Tomiyama K, Mori A, et al. Section 7. A new therapeutic strategy on portal flow modulation that increases donor safety with good recipient outcomes. Transplantation. 2014;97:30–2.
Yao S, Kaido T, Uozumi R, Yagi S, Miyachi Y, Fukumitsu K, et al. Is portal venous pressure modulation still indicated for all recipients in living donor liver transplantation? Liver Transpl. 2018;24:1578–88.
Kuriyama N, Iizawa Y, Kato H, Murata Y, Tanemura A, Azumi Y, et al. Impact of splenectomy just before partial orthotopic liver transplantation using small-for-size graft in rats. Transplant Proc. 2016;48:1304–8.
Ito K, Akamatsu N, Ichida A, Ito D, Kaneko J, Arita J, et al. Splenectomy is not indicated in living donor liver transplantation. Liver Transpl. 2016;22:1526–35.
Ikegami T, Toshima T, Takeishi K, Soejima Y, Kawanaka H, Yoshizumi T, et al. Bloodless splenectomy during liver transplantation for terminal liver diseases with portal hypertension. J Am Coll Surg. 2009;208:e1–4.
Linares I, Goldaracena N, Rosales R, Maza L, Kaths M, Kollmann D, et al. Splenectomy as flow modulation strategy and risk factors of de novo portal vein thrombosis in adult-to-adult living donor liver transplantation. Liver Transpl. 2018;24:1209–20.
Sanchez-Bueno F, Hernandez Q, Ramirez P, Robles R, Acosta F, Rodriguez JM, et al. Vascular complications in a series of 300 orthotopic liver transplants. Transplant Proc. 1999;31:2409–10.
Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896–903.
Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, et al. Portal vein complications after adult-to-adult living donor liver transplantation. Transpl Int. 2008;21:1136–44.
Sugawara Y, Makuuchi M, Tamura S, Matsui Y, Kaneko J, Hasegawa K, et al. Portal vein reconstruction in adult living donor liver transplantation using cryopreserved vein grafts. Liver Transpl. 2006;12:1233–6.
Kawanaka H, Akahoshi T, Itoh S, Iguchi T, Harimoto N, Uchiyama H, et al. Optimizing risk stratification in portal vein thrombosis after splenectomy and its primary prophylaxis with antithrombin III concentrates and danaparoid sodium in liver cirrhosis with portal hypertension. J Am Coll Surg. 2014;219:865–74.
Kinjo N, Kawanaka H, Akahoshi T, Tomikawa M, Yamashita N, Konishi K, et al. Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension. Br J Surg. 2010;97:910–6.
Kurata N, Ogura Y, Ogiso S, Onishi Y, Kamei H, Kodera Y. Splenectomy in living donor liver transplantation and risk factors of portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2019;18:337–42.
Badawy A, Hamaguchi Y, Satoru S, Kaido T, Okajima H, Uemoto S. Evaluation of safety of concomitant splenectomy in living donor liver transplantation: a retrospective study. Transpl Int. 2017;30:914–23.
Matsukura A, Kita Y, Harihara Y, Kubota K, Takayama T, Kawarasaki H, et al. Is splenic artery ligation effective for thrombocytopenia early after liver transplantation? Transplant Proc. 1999;31:2906–7.
Troisi R, Hoste E, Van Langenhove P, Decruyenaere J, Voet D, Hesse UJ, et al. Modulation of liver graft hemodynamics by partial ablation of the splenic circuit: a way to increase hepatic artery flow? Transplant Proc. 2001;33:1445–6.
Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, et al. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429–36.
Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626–8.
Ishizaki Y, Kawasaki S, Sugo H, Yoshimoto J, Fujiwara N, Imamura H. Left lobe adult-to-adult living donor liver transplantation: Should portal inflow modulation be added? Liver Transpl. 2012;18:305–14.
Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, et al. Preoperative proximal splenic artery embolization: a safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl Int. 2007;20:947–55.
Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, et al. Effects of prophylactic splenic artery modulation on portal overperfusion and liver regeneration in small-for-size graft. Transplantation. 2008;86:673–80.
Moon DB, Lee SG, Hwang S, Ahn CS, Kim KH, Ha TY, et al. Splenic devascularization can replace splenectomy during adult living donor liver transplantation - a historical cohort study. Transpl Int. 2019;32:535–45.
Zhou HY, Chen TW, Zhang XM, Jing ZL, Zeng NL, Zhai ZH. Patterns of portosystemic collaterals and diameters of portal venous system in cirrhotic patients with hepatitis B on magnetic resonance imaging: Association with Child-Pugh classifications. Clin Res Hepatol Gastroenterol. 2015;39:351–8.
Reddy MS, Rela M. Portosystemic collaterals in living donor liver transplantation: What is all the fuss about? Liver Transpl. 2017;23:537–44.
Sanchez-Cabus S, Fondevila C, Calatayud D, Ferrer J, Taura P, Fuster J, et al. Importance of the temporary portocaval shunt during adult living donor liver transplantation. Liver Transpl. 2013;19:174–83.
De Carlis L, Del Favero E, Rondinara G, Belli LS, Sansalone CV, Zani B, et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl Int. 1992;5:9–14.
Moon DB, Lee SG, Ahn C, Hwang S, Kim KH, Ha T, et al. Application of intraoperative cine-portogram to detect spontaneous portosystemic collaterals missed by intraoperative doppler exam in adult living donor liver transplantation. Liver Transpl. 2007;13:1279–84.
Moon DB, Lee SG, Kim KH, Ahn CS, Hwang S, Park KM, et al. The significance of complete interruption of large spontaneous portosystemic collaterals in adult living donor liver transplantation as a graft salvage procedure. Transpl Int. 2008;21:698–700.
Ikegami T, Shirabe K, Nakagawara H, Yoshizumi T, Toshima T, Soejima Y, et al. Obstructing spontaneous major shunt vessels is mandatory to keep adequate portal inflow in living-donor liver transplantation. Transplantation. 2013;95:1270–7.
Nagao Y, Akahoshi T, Uehara H, Hashimoto N, Kinjo N, Kawanaka H, et al. Balloon-occluded retrograde transvenous obliteration is feasible for prolonged portosystemic shunts after living donor liver transplantation. Surg Today. 2014;44:633–9.
Takatsuki M, Baimakhanov Z, Soyama A, Inoue Y, Hidaka M, Kuroki T, et al. Obstructing spontaneous major shunt vessels might not be mandatory to maintain adequate portal inflow in living donor liver transplantation. Transplantation. 2014;97:e52–e5353.
Boillot O, Delafosse B, Mechet I, Boucaud C, Pouyet M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406–7.
Asakura T, Ohkohchi N, Orii T, Koyamada N, Tsukamoto S, Sato M, et al. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl Int. 2003;16:376–82.
Wang HS, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Asakura T, et al. Excessive portal flow causes graft failure in extremely small-for-size liver transplantation in pigs. World J Gastroenterol. 2005;11:6954–9.
Takada Y, Ueda M, Ishikawa Y, Fujimoto Y, Miyauchi H, Ogura Y, et al. End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2004;10:807–10.
Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, et al. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397–404.
Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16:649–57.
Oura T, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Uno M, et al. Does the permanent portacaval shunt for a small-for-size graft in a living donor liver transplantation do more harm than good? Am J Transplant. 2008;8:250–2.
Ozden I, Imura S. Somatostatin and propranolol for the treatment of small-for-size syndrome after liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:560–1.
Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102.
Xu X, Man K, Zheng SS, Liang TB, Lee TK, Ng KT, et al. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transpl. 2006;12:621–7.
Hessheimer AJ, Escobar B, Munoz J, Flores E, Gracia-Sancho J, Taura P, et al. Somatostatin therapy protects porcine livers in small-for-size liver transplantation. Am J Transplant. 2014;14:1806–16.
Troisi RI, Vanlander A, Giglio MC, Van Limmen J, Scudeller L, Heyse B, et al. Somatostatin as inflow modulator in liver-transplant recipients with severe portal hypertension: a randomized trial. Ann Surg. 2019;269:1025–33.
Kawachi S, Shimazu M, Wakabayashi G, Tanabe M, Shirasugi N, Kumamoto Y, et al. Efficacy of intraportal infusion of prostaglandin E1 to improve the hepatic blood flow and graft viability in porcine liver transplantation. Transplantation. 1997;64:205–9.
Imura S, Shimada M, Ikegami T, Morine Y, Kanemura H. Strategies for improving the outcomes of small-for-size grafts in adult-to-adult living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:102–10.
Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, et al. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int. 2005;18:923–8.
Di Domenico S, Andorno E, Varotti G, Valente U. Hepatic flow optimization in full right split liver transplantation. World J Gastrointest Surg. 2011;3:110–202.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshizumi, T., Mori, M. Portal flow modulation in living donor liver transplantation: review with a focus on splenectomy. Surg Today 50, 21–29 (2020). https://doi.org/10.1007/s00595-019-01881-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01881-y