Abstract
Purpose
Early detection of a response to neoadjuvant chemotherapy for locally advanced rectal cancer may spare patients from additional toxic but ineffective chemotherapy. Using 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), we evaluated tumor response prospectively in the early course of preoperative chemotherapy.
Methods
The subjects were 15 patients who received neoadjuvant chemotherapy (XELOX or XELOX plus bevacizumab) for locally advanced rectal cancer. Patients underwent 18F-FDG PET before chemotherapy, at the end of the first cycle of chemotherapy, and before surgical resection. Magnetic resonance imaging (MRI) was performed before chemotherapy, after the second cycle of chemotherapy, and before resection. After resection, the SUVmax and diameter were compared and graded according to the tumor regression grade (TRG).
Results
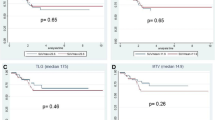
The TRG was assessed as TRG1 in one patient, TRG2 in five patients, and TRG3 in nine patients. We divided the patients into two groups: non-responders (NR) included the TRG1 and TRG2 patients, and responders (R) included the TRG3 patients. The tumor size before surgery was significantly smaller in the R group than in the NR group. The SUVmax at the end of the first cycle of chemotherapy and before surgical resection was significantly lower in the R group than in the NR group.
Conclusion
Performing 18F-FDG PET at the end of the first cycle of chemotherapy allowed us to predict the pathological response of locally advanced rectal cancer.



Similar content being viewed by others
References
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–82.
Hida J, Okuno K, Tokoro T. Distal dissection in total mesorectal excision, and preoperative chemoradiotherapy and lateral lymph node dissection for rectal cancer. Surg Today. 2014;44(12):2227–42.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60.
Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007;2:CD002102.
Uehara K, Nagino M. Neoadjuvant treatment for locally advanced rectal cancer: a systematic review. Surg Today. 2015. doi:10.1007/s00595-015-1218-z.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW, et al. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14(10):2773–9.
van Duijvendijk P, Slors JF, Taat CW, van Tets WF, van Tienhoven G, Obertop H, et al. Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol. 2002;97(9):2282–9.
Birgisson H, Pahlman L, Gunnarsson U, Glimelius B, Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23(34):8697–705.
Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–206.
Hasegawa J, Nishimura J, Mizushima T, Miyake Y, Kim HM, Takemoto H, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73(5):1079–87.
Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5.
Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8.
Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: n-SOG 03 phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71.
Capirci C, Rubello D, Chierichetti F, Crepaldi G, Fanti S, Mandoliti G, et al. Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. AJR Am J Roentgenol. 2006;187(2):W202–8.
Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7.
Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47(1):14–22.
Leibold T, Akhurst TJ, Chessin DB, Yeung HW, Macapinlac H, Shia J, et al. Evaluation of (1)(8)F-FDG-PET for early detection of suboptimal response of rectal cancer to preoperative chemoradiotherapy: a prospective analysis. Ann Surg Oncol. 2011;18(10):2783–9.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688–96.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–12.
Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–91.
Yoshida Y, Hoshino S, Aisu N, Naito M, Tanimura S, Mogi A, et al. Administration of chemotherapy via the median cubital vein without implantable central venous access ports: port-free chemotherapy for metastatic colorectal cancer patients. Int J Clin Oncol. 2015;20(2):332–7.
Yoshida Y, Hoshino S, Aisu N, Mogi A, Yamada T, Kojima D, et al. Can grade 2 neutropenia predict the risk of grade 3 neutropenia in metastatic colorectal cancer patients treated with chemotherapy? Support Care Cancer. 2015;23(6):1623–7.
Gosens MJ, Dresen RC, Rutten HJ, Nieuwenhuijzen GA, van der Laak JA, Martijn H, et al. Preoperative radiochemotherapy is successful also in patients with locally advanced rectal cancer who have intrinsically high apoptotic tumours. Ann Oncol. 2008;19(12):2026–32.
Lange MM, den Dulk M, Bossema ER, Maas CP, Peeters KC, Rutten HJ, et al. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94(10):1278–84.
Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23(9):1847–58.
Pollack J, Holm T, Cedermark B, Holmstrom B, Mellgren A. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49(3):345–52.
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43(1):189–204.
Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250(3):730–9.
Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260(3):734–43.
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010;252(6):998–1004.
Musio D, De Felice F, Magnante AL, Ciolina M, De Cecco CN, Rengo M, et al. Diffusion-weighted magnetic resonance application in response prediction before, during, and after neoadjuvant radiochemotherapy in primary rectal cancer carcinoma. Biomed Res Int. 2013;2013:740195.
Nougaret S, Fujii S, Addley HC, Bibeau F, Pandey H, Mikhael H, et al. Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: Initial experience. J Magn Reson Imaging. 2013;38(3):726–32.
Aiba T, Uehara K, Nihashi T, Tsuzuki T, Yatsuya H, Yoshioka Y, et al. MRI and FDG-PET for assessment of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2014;21(6):1801–8.
Guerra L, Niespolo R, Di Pisa G, Ippolito D, De Ponti E, Terrevazzi S, et al. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging. 2011;36(1):38–45.
Acknowledgments
This study was supported by the Osaka Medical Research Foundation for Incurable Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Nishimura, J., Hasegawa, J., Ogawa, Y. et al. 18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET) for the early detection of response to neoadjuvant chemotherapy for locally advanced rectal cancer. Surg Today 46, 1152–1158 (2016). https://doi.org/10.1007/s00595-015-1297-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1297-x