Abstract
Aims
Accumulating evidences indicate that abnormalities in tubular lipid metabolism play a crucial role in the development of diabetic kidney disease (DKD). We aim to identify novel lipid metabolism-related genes associated with tubular injury in DKD by utilizing bioinformatics approaches.
Methods
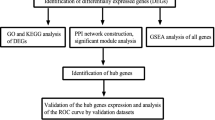
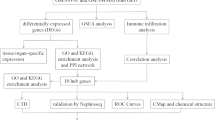
Differentially expressed genes (DEGs) between control and DKD tubular tissue samples were screened from the Gene Expression Omnibus (GEO) database, and then were intersected with lipid metabolism-related genes. Hub genes were further determined by combined weighted gene correlation network analysis (WGCNA) and protein–protein interaction (PPI) network. We performed enrichment analysis, immune analysis, clustering analysis, and constructed networks between hub genes and miRNAs, transcription factors and small molecule drugs. Receiver operating characteristic (ROC) curves were employed to evaluate the diagnostic efficacy of hub genes. We validated the relationships between hub genes and DKD with external datasets and our own clinical samples.
Results
There were 5 of 37 lipid metabolism-related DEGs identified as hub genes. Enrichment analysis demonstrated that lipid metabolism-related DEGs were enriched in pathways such as peroxisome proliferator-activated receptors (PPAR) signaling and pyruvate metabolism. Hub genes had potential regulatory relationships with a variety of miRNAs, transcription factors and small molecule drugs, and had high diagnostic efficacy. Immune infiltration analysis revealed that 13 immune cells were altered in DKD, and hub genes exhibited significant correlations with a variety of immune cells. Through clustering analysis, DKD patients could be classified into 3 immune subtypes and 2 lipid metabolism subtypes, respectively. The tubular expression of hub genes in DKD was further verified by other external datasets, and immunohistochemistry (IHC) staining showed that except ACACB, the other 4 hub genes (LPL, AHR, ME1 and ALOX5) exhibited the same results as the bioinformatics analysis.
Conclusion
Our study identified several key lipid metabolism-related genes (LPL, AHR, ME1 and ALOX5) that might be involved in tubular injury in DKD, which provide new insights and perspectives for exploring the pathogenesis and potential therapeutic targets of DKD.










Similar content being viewed by others
Data availability
The datasets presented in the current study can be found in online repositories. The experimental data are available from the corresponding author upon reasonable request.
References
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12(12):2032–2045
Yang W, Luo Y, Yang S et al (2018) Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin Sci (Lond) 132(22):2407–2422
Kimmelstiel P, Wilson C (1936) Intercapillary lesions in the glomeruli of the Kidney. Am J Pathol 12(1):83–98
Bai Y, Ma L, Deng D et al (2023) Bioinformatic identification of genes involved in diabetic nephropathy fibrosis and their clinical relevance. Biochem Genet 61(4):1567–1584
Mai L, He G, Chen J et al (2023) Profilin1 promotes renal tubular epithelial cell apoptosis in diabetic nephropathy through the Hedgehog signaling pathway. Diabetes Metab Syndr Obes 16:1731–1743
Bai F, Yu K, Yang Y et al (2022) Identification and validation of P4HB as a novel autophagy-related biomarker in diabetic nephropathy. Front Genet 13:965816
Ma LL, Bai Y, Liu WH et al (2023) Bioinformatics analysis of potential key ferroptosis-related genes involved in tubulointerstitial injury in patients with diabetic nephropathy. Ren Fail 45(1):2199095
Zhao J, He K, Du H et al (2022) Bioinformatics prediction and experimental verification of key biomarkers for diabetic kidney disease based on transcriptome sequencing in mice. PeerJ 10:e13932
Liberzon A, Birger C, Thorvaldsdóttir H et al (2015) The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1(6):417–425
Huang HY, Lin YC, Li J et al (2020) miRTarBase 2020: updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res 48(D1):D148–D154
Davis CA, Hitz BC, Sloan CA et al (2018) The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res 46(D1):D794–D801
Zhang B, Wu Q, Li B et al (2020) m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer 19(1):53
Wang H, Zhang S, Guo J (2021) Lipotoxic proximal tubular injury: a primary event in diabetic kidney disease. Front Med (Lausanne) 8:751529
Li J, Li L, Guo D et al (2020) Triglyceride metabolism and angiopoietin-like proteins in lipoprotein lipase regulation. Clin Chim Acta 503:19–34
Eu CH, Lim WY, Ton SH et al (2010) Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis 9:81
Liu Y, Wang ZB, Yin WD et al (2011) Preventive effect of Ibrolipim on suppressing lipid accumulation and increasing lipoprotein lipase in the kidneys of diet-induced diabetic minipigs. Lipids Health Dis 10:117
Bock KW (2019) Functions of aryl hydrocarbon receptor (AHR) and CD38 in NAD metabolism and nonalcoholic steatohepatitis (NASH). Biochem Pharmacol 169:113620
Gutierrez-Vazquez C, Quintana FJ (2018) Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48(1):19–33
Murray IA, Patterson AD, Perdew GH (2014) Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer 14(12):801–814
Dou L, Poitevin S, Sallée M et al (2018) Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int 93(4):986–999
Kim JT, Kim SS, Jun DW et al (2013) Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig 4(5):483–491
Miao H, Wu XQ, Wang YN et al (2022) 1-Hydroxypyrene mediates renal fibrosis through aryl hydrocarbon receptor signalling pathway. Br J Pharmacol 179(1):103–124
Lee WJ, Liu SH, Chiang CK et al (2016) Aryl hydrocarbon receptor deficiency attenuates oxidative stress-related mesangial cell Activation and macrophage infiltration and extracellular matrix accumulation in diabetic nephropathy. Antioxid Redox Signal 24(4):217–231
Zhu XY, Xia HG, Wang ZH et al (2020) In vitro and in vivo approaches for identifying the role of aryl hydrocarbon receptor in the development of nonalcoholic fatty liver disease. Toxicol Lett 319:85–94
Rojas IY, Moyer BJ, Ringelberg CS et al (2020) Reversal of obesity and liver steatosis in mice via inhibition of aryl hydrocarbon receptor and altered gene expression of CYP1B1, PPARalpha, SCD1, and osteopontin. Int J Obes (Lond) 44(4):948–963
Yang X, Deignan JL, Qi H et al (2009) Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet 41(4):415–423
Rui L (2014) Energy metabolism in the liver. Compr Physiol 4(1):177–197
López IP, Marti A, Milagro FI et al (2003) DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes Res 11(2):188–194
Kondo H, Minegishi Y, Komine Y et al (2006) Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am J Physiol Endocrinol Metab 291(5):E1092–1099
Al-Dwairi A, Pabona JM, Simmen RC et al (2012) Cytosolic malic enzyme 1 (ME1) mediates high fat diet-induced adiposity, endocrine profile, and gastrointestinal tract proliferation-associated biomarkers in male mice. PLoS ONE 7(10):e46716
Zhong H, Beaulaurier J, Lum PY et al (2010) Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet 6(5):e1000932
Martínez-Clemente M, Clària J, Titos E (2011) The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care 14(4):347–353
Montford JR, Bauer C, Dobrinskikh E et al (2019) Inhibition of 5-lipoxygenase decreases renal fibrosis and progression of chronic kidney disease. Am J Physiol Renal Physiol 316(4):F732–F742
Chen X, Xie H, Liu Y et al (2023) Interference of ALOX5 alleviates inflammation and fibrosis in high glucose-induced renal mesangial cells. Exp Ther Med 25(1):34
Chen L, Duan Y, Wei H et al (2019) Acetyl-CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert Opin Investig Drugs 28(10):917–930
Cho YS, Lee JI, Shin D et al (2010) Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK. Biochem Biophys Res Commun 391(1):187–192
Xu Y, Huang J, Xin W et al (2014) Lipid accumulation is ahead of epithelial-to-mesenchymal transition and therapeutic intervention by acetyl-CoA carboxylase 2 silence in diabetic nephropathy. Metabolism 63(5):716–726
Xin W, Zhao X, Liu L et al (2015) Acetyl-CoA carboxylase 2 suppression rescues human proximal tubular cells from palmitic acid induced lipotoxicity via autophagy. Biochem Biophys Res Commun 463(3):364–369
Gervois P, Torra IP, Fruchart JC et al (2000) Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med 38(1):3–11
Tesch GH (2017) Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond) 131(16):2183–2199
Moon JY, Jeong KH, Lee TW et al (2012) Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol 35(2):164–174
Zhang F, Wang C, Wen X et al (2020) Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103(+) DCs-mediated CD8(+) T cell responses. J Cell Mol Med 24(10):5817–5831
Acknowledgements
We acknowledge the GEO database and Nephroseq database contributors for sharing the meaningful data to the general public.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No: 81860161), the science and technology fund projects of Guizhou health committee [gzwjkj2020-1–003], the funding for provincial key medical discipline construction project of Health Commission of Guizhou Province from 2023 to 2024, and Guizhou Provincial Science and Technology Department Project (Grant No: qian ke he jichu-ZK[2022]yiban 448).
Author information
Authors and Affiliations
Contributions
JH designed the study. YF performed the bioinformatics, experiment, data gathering, and draft writing. JH executed the data processing and statistical analysis. LS revised the manuscript and executed supervision throughout the process. MZ gave advice on the data analysis. LX made suggestions on sample selection. YC, NH and YJ coordinated the experiment and data gathering. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study involving human participants was performed in accordance with the 1964 Helsinki Declaration and its later amendments, and approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University (approval No. 2023-328).
Informed consent
The patients/participants provided their written informed consent to participate in this study.
Additional information
Managed by Giuseppe Pugliese .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., He, J., Shi, L. et al. Identification of potential key lipid metabolism-related genes involved in tubular injury in diabetic kidney disease by bioinformatics analysis. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02278-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02278-1