Abstract
Aims
To determine whether carotid intima-media thickness (CIMT), a surrogate marker of cardiovascular disease (CVD), is associated with long-term blood glucose control in individuals with type 1 diabetes (T1D).
Methods
We recruited 508 individuals (43.4% men; median age 46.1, IQR 37.8–55.9 years) with T1D (median diabetes duration of 30.4, IQR 21.2–40.8 years) in a cross-sectional retrospective sub-study, part of the Finnish Diabetic Nephropathy (FinnDiane) Study. Glycated hemoglobin (HbA1c) data were collected retrospectively over the course of ten years (HbA1c-meanoverall) prior to the clinical study visit that included a clinical examination, biochemical sampling, and ultrasound of the common carotid arteries.
Results
Individuals with T1D had a median CIMT of 606 μm (IQR 538–683 μm) and HbA1c of 8.0% (7.3–8.8%) during the study visit and HbA1c-meanoverall of 8.0% (IQR 7.3–8.8%). CIMT did not correlate with HbA1c (p = 0.228) at visit or HbA1c-meanoverall (p = 0.063). After controlling for relevant factors in multivariable linear regression analysis, only age was associated with CIMT (p < 0.001). After further dividing CIMT into quartiles, no correlation between long-term glucose control and CIMT (%, 1st 8.1 [IQR 7.2–8.9] vs 4th 7.9 [7.4–8.7], p = 0.730) was found.
Conclusions
We observed no correlation between long-term blood glucose control and CIMT in individuals with T1D. This finding suggests that the development of early signs of macrovascular atherosclerosis is not strongly affected by the glycemic control in people with T1D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotid intima-media thickness (CIMT), a surrogate marker of atherosclerosis, predicts cardiovascular events, particularly myocardial infarction, and stroke. CIMT can be measured using ultrasound of the two innermost layers of the carotid artery wall, tunica intima and tunica media. Ultrasound of the CIMT provides an easy, reproducible non-invasive tool to assess the risk of cardiovascular disease (CVD). It can be measured by a trained technician with a mobile ultrasound device in an outpatient setting [1].
Type 1 diabetes (T1D) increases the risk of CVD [2], and individuals with T1D have a sixfold increased risk of stroke compared to non-diabetic individuals [3]. It is of note that these T1D individuals have increased CIMT compared to healthy controls [1, 4]. We recently demonstrated that CIMT is a potential indicator of cerebral small vessel disease in individuals with T1D, regardless of blood glucose control [5].
Surprisingly, data on the effect of blood glucose control on CIMT in T1D are short. The Epidemiology of Diabetes Interventions and Complications (EDIC) trial, a long-term follow-up of the Diabetes Control and Complications Trial (DCCT), demonstrated that intensive blood glucose treatment of T1D had slowed the progression of CIMT as observed 6 years after the end of the intervention compared to the conventional blood glucose treatment. [6, 7] CIMT correlated with the age and duration of diabetes in a cross-sectional study in adolescents and young adult individuals with T1D, regardless of other clinical covariates. Furthermore, there was a non-significant trend between sex and blood glycated hemoglobin (HbA1c) and CIMT. [8] Larsen and colleagues observed an independent association between HbA1c and CIMT in women; however, no correlation was seen among men [9].
We aimed to explore the relationship between CIMT and the current glycemic control, as well as the long-term glycemic control over the preceding ten years, in a well-characterized cohort of Finnish individuals with T1D. The findings of our study may shed light on the underlying metabolic mechanisms at play, potentially building rationales for future research in the field.
Methods
Study population
This research was conducted as part of the Finnish Diabetic Nephropathy (FinnDiane) Study, a comprehensive, nationwide, multicenter study that aims to uncover genetic, environmental, and clinical risk factors for micro- and macrovascular complications of T1D. The final aim of the FinnDiane study is to understand underlying causes of diabetes-related complications and to identify potential targets for intervention and prevention. The FinnDiane study protocol has been published previously [10].
For this substudy, a total of 508 individuals with T1D with an onset of diabetes < 40 years and age span ranging from 19.5 to 80.8 years were included. All participants were consecutively enrolled at the FinnDiane Study Center at the Helsinki University Hospital between 2009 and 2019.
Ethical considerations
The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District. Each participant signed a written informed consent.
Laboratory tests and clinical examination
Blood samples were drawn for the determination of serum lipids and lipoproteins (total cholesterol, high-density lipoprotein [HDL] cholesterol and triglycerides), serum creatinine, HbA1c, and high-sensitivity C-reactive protein (hs-CRP). The Friedewald equation was used to calculate the low-density lipoprotein (LDL) cholesterol concentration [11]. The CKD-EPI-formula was used to calculate the estimated glomerular filtration rate (eGFR) [12]. Hypertension was defined as office measurement of systolic blood pressure (SBP) ≥ 140, diastolic blood pressure (DBP) ≥ 90, or the use of anti-hypertensive medication.
Albuminuria was defined by the urinary albumin excretion rate (UAER) ≥ 20 μg/min or UAER ≥ 30 mg/24 h in two out of three consecutive overnight or 24-h urine collections. Individuals (n = 57) on kidney replacement therapy (dialysis or kidney transplant) were included in study but excluded from the albuminuria analysis. Of the individuals, 32 had missing albuminuria data.
Medical records and in-depth questionnaires, previously described, were part of the clinical data [13]. Smoking was defined as current or history of smoking at least one cigarette per day for at least one year. History of retinal photocoagulation was used as a marker of severe diabetic retinopathy. Coronary heart disease was defined as the diagnosis of myocardial infarction or history of coronary revascularization. Stroke was defined as either cerebral infarction or intracerebral hemorrhage. Peripheral vascular disease was defined as history of revascularization of a peripheral artery or lower limb amputation. In this study, cardiovascular events included the diagnosis of myocardial infarction, the need for coronary revascularization, stroke, or peripheral vascular disease.
Measures of glycemic control
For current glycemic control, we used the level of HbA1c, a biomarker that indicates the average blood glucose control over a period of two to three months [14]. HbA1c was measured using standardized assays in a central laboratory (Medix Laboratories, Espoo Finland).
To gain a broader picture of long-term glucose control, we obtained at least five HbA1c values for each individual over a period of ten years preceding the study visit. These values were used to calculate the overall mean HbA1c (HbA1c-meanoverall). These HbA1c values were collected from the medical files and had been determined by standardized methods, high-performance liquid chromatography (HPLC), with a reference range of 4–6% (20–42 mmol/mol).
Measurement of carotid intima-media thickness
Ultrasound imaging of the carotid arteries was conducted on both the left and right sides. The distal 1-cm segment of the common carotid artery, immediately preceding the point of origin of the bulb, was scanned by a trained nurse, using a specialized ultrasound scanner (MyLab 70, Esaote, Genova, Italy) equipped with a 10-MHz linear probe. The mean of two measurements of the left and right CIMT was calculated. [6, 15]
The scanner was integrated with a radiofrequency-based tracking of the arterial wall (QIMT®), which enables semi-automatic and real-time determination of CIMT values during six cardiac cycles of the far-wall CIMT [16, 17].
Statistics
Statistical analysis was done with IBM SPSS Statistics 27.0 (IBM, Armonk, NY, USA). Kruskal–Wallis tests were used for the nonparametric data presented as medians (interquartile range). The X2 test was used for categorical variables. Unadjusted linear regression models were used to analyze relationship between CIMT and HbA1c, HbA1c-meanoverall, and age. To analyze the relationship between CIMT and HbA1c, HbA1c-meanoverall, and clinically relevant risk factors (age, sex, history of cardiovascular event, history of retinal photocoagulation, systolic blood pressure, use of lipid lowering drug, and eGFR) that differed between CIMT quartiles, multivariable linear regression models were built with the CIMT as dependent variable and HbA1c or HbA1c-meanoverall, and clinically relevant risk factors as independent variables. The threshold for statistical significance was set at p < 0.05.
Results
Clinical characteristics
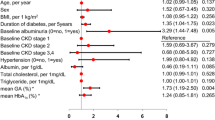
Five hundred and eight individuals with T1D were included in this study, and their clinical characteristics are presented in Table 1. Median age was 46.1 (IQR 37.8–55.9) years and 43.3% were men. The diabetes duration was 30.4 (IQR 21.2–40.8) years. Sixty-nine individuals had a history of a cardiovascular event of which 22 had had a stroke. Two hundred and five individuals had had retinal laser photocoagulation. Systolic blood pressure was 134 mmHg (IQR 122–147 mmHg) and diastolic 76 mmHg (IQR 70–83 mmHg). Two hundred and sixty-nine individuals were taking medication for high blood pressure. The HbA1c at the time of the CIMT measurement was 8.0% (7.3–8.8%), (64 mmol/mol [56–72 mmol/mol]), while the HbA1c-meanoverall (median count 18, IQR 13–28) was 8.0% (IQR 7.3–8.8%), (64 mmol/mol [IQR 57–72 mmol/mol]). The clinical characteristics are presented in Table 1.
CIMT
Study participants had a median CIMT value of 606 μm (IQR 538–683 μm). CIMT did not correlate with HbA1c at the time of the study visit (Fig. 1). Although there was a trend toward a correlation between the HbA1c-meanoverall and CIMT, it did not reach statistical significance (p = 0.063) (Fig. 2). Age was positively correlated with CIMT (Fig. 3).
CIMT quartiles
Individuals were further divided into quartiles based on CIMT values. CIMT quartiles correlated with age and diabetes duration, while no correlation was observed between CIMT quartiles, sex, history of smoking, or BMI (Table 1).
Furthermore, systolic and diastolic blood pressure, use of anti-hypertensive drugs, use of renin–angiotensin–aldosterone system blockers correlated with CIMT quartiles. LDL cholesterol correlated inversely and the use of lipid-lowering drugs correlated positively with CIMT quartiles as well. No correlations were observed between CIMT quartiles and total cholesterol, HDL cholesterol, triglycerides, or hs-CRP (Table 1).
We further investigated the association between CIMT and diabetic kidney disease. Of note eGFR but not albuminuria correlated with CIMT quartiles (Table 1). Furthermore, a history of a cardiovascular event, stroke, coronary heart disease, peripheral vascular disease and retinal photocoagulation correlated with CIMT quartiles (Table 1).
HbA1c levels at the time of the study did not correlate with CIMT quartiles in individuals with T1D. Finally, there was no correlation between the CIMT quartiles and HbA1c-meanoverall either (Table 1).
In multivariable linear regression analysis with CIMT as the dependent variable including HbA1c or HbA1c-meanoverall, age, sex, history of cardiovascular events, retinal photocoagulation, systolic blood pressure, the use of lipid-lowering drug, and estimated glomerular filtration rate, age was the only covariate that remained significantly associated with CIMT (Table 2). There was no observed correlation in the multivariable linear regression analysis, even after excluding individuals who had a history of cardiovascular events (Supplementary Table 1).
Discussion
The main finding of our study was the lack of association between long-term blood glucose control and CIMT in individuals with T1D. The finding was surprising considering previous data showing associations between blood glucose control and CIMT in other cohorts of people with T1D. Only age was independently associated with CIMT.
Several variables were significantly correlated with CIMT after dividing the variable into quartiles. These factors were age, duration of diabetes, history of cardiovascular events, history of retinal photocoagulation, systolic blood pressure/anti-hypertensive medication, and eGFR. In contrast, LDL cholesterol or lipid-lowering medication showed a reverse relationship with CIMT, suggesting that older individuals with elevated CIMT and co-existing conditions were adequately treated with lipid-lowering medication.
Our multivariable linear regression analysis revealed that only age was independently associated with CIMT. The association between age and CIMT has been consistently observed across multiple studies involving different populations, such as the general population, and those with type 2 diabetes and chronic kidney disease, among others [18, 19].
At the time of the study visit, there was no observed correlation between CIMT and HbA1c or HbA1c-meanoverall. Although there was a tendency for HbA1c-meanoverall and CIMT to show an inverse correlation, this trend did not reach statistical significance (Fig. 2). It is possible that this trend may be attributed to the fact that older individuals with higher CIMT and longstanding diabetes may have established a more optimal glucose control, as opposed to younger patients who are still striving to achieve optimal diabetes control.
The DCCT/EDIC study investigated the impact of intensive insulin therapy on the progression of microvascular and macrovascular complications in individuals T1D. The results of the DCCT/EDIC study showed that intensive insulin therapy resulted in improved glucose control that was associated with slower progression of CIMT. The reason why our study did not yield similar results as the DCCT/EDIC, may be that our study was cross-sectional and had a retrospective design, while the DCCT/EDIC study was a randomized control trial. However, other factors may also contribute to the disparities. For instance, individuals in the DCCT/EDIC may have been more intensively managed, not only with respect to their blood glucose control, but also regarding lifestyle and pharmacologic treatment. However, our cohort presents real-time data on individuals with T1D in Finland. Additionally, the current epidemic of obesity and the co-occurrence of T1D and metabolic syndrome, known as hybrid diabetes, or presence of fatty liver disease may further complicate the relationship between CIMT and T1D in our study population [10, 20, 21].
Interestingly, no association was observed between CIMT and coronary artery events in the DCCT/EDIC, although intensive insulin therapy with subsequently improved glucose control was shown to reduce the risk of CVD in that trial [22]. Lastly, CIMT has not been shown to associate with cardiovascular events in individuals with T1D [6, 7, 23].
There is limited knowledge available regarding the relationship between T1D and CIMT. CIMT is a biomarker of atherosclerosis [1]. In the context of T1D, the pathogenesis of arterial disease is thought to involve calcification, endothelial dysfunction, and arterial stiffness. The role of atherosclerosis in arterial disease in these individuals is thought to be different compared to people without diabetes [24, 25]. Furthermore, individuals with the metabolic syndrome included in the study may partly explain the connection between CIMT and arterial disease more than hyperglycemia per se. This is an area for speculation and further investigation. It is possible that various cell-level factors may play a role in the advancement of CIMT in individuals with T1D. Some of these potential contributors include oxidative stress, chronic inflammation, the activation of the renin–angiotensin–aldosterone system, and the activation of the endoplasmic reticulum stress pathway [24, 26]. In our cross-sectional study, hs-CRP, a marker of chronic inflammation, did not correlate with CIMT. Overall, these findings highlight the complexity of the relationship between T1D, arterial disease, and atherosclerosis. Further research is needed to better understand the underlying mechanisms driving the progression of CIMT in T1D.
Our study has certain limitations that should be acknowledged. We did not collect data on short-term glucose control measures such as time in range (TIR) or variability obtained from continuous glucose monitoring systems (CGMS), which represents an area of interest for future studies to explore. No causal relationships can be explored given the cross-sectional nature of our study. However, it should be noted that blood glucose values from a ten-year period prior to the study visit were analyzed, which allowed for the assessment of cumulative and long-term blood glucose control.
Despite these limitations, our study has notable strengths that contribute to the understanding of CIMT in T1D. The cohort allowed robust analyses. Additionally, the use of standardized imaging and clinical assessments is a notable strength of our study, which enhances the reliability and validity of our results.
Conclusion
We showed that long-term blood glucose control does not associate with CIMT in people with T1D. Age was the only factor independently associated with CIMT. This result differs from previous findings showing blood glucose control to be related to CIMT in T1D. Our findings, thus, suggest that there are other factors involved. We hope that our findings will pave the way for future studies that can further explore and shed light on the complex interplay of various factors in determining CIMT in T1D.
Data availability
Individual-level data of the study participants are not publicly available because of the restrictions due to the study consent provided by the participant at the time of data collection. Readers may, however, request collaboration with the authors to explore individual-level data by contacting the lead investigator.
References
Sibal L, Agarwal SC, Home PD (2011) Carotid intima-media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab Syndr Obes 4:23–34. https://doi.org/10.2147/DMSO.S8540
Rawshani A, Rawshani A, Franzen S, et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376(15):1407–1418. https://doi.org/10.1056/NEJMoa1608664
Harjutsalo V, Pongrac Barlovic D, Groop PH (2021) Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: a retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol 9(9):575–585. https://doi.org/10.1016/S2213-8587(21)00172-8
Sun YP, Cai YY, Li HM, Deng SM, Leng RX, Pan HF (2015) Increased carotid intima-media thickness (CIMT) levels in patients with type 1 diabetes mellitus (T1DM): A meta-analysis. J Diabetes Complications 29(5):724–730. https://doi.org/10.1016/j.jdiacomp.2015.03.018
Inkeri J, Tynjälä A, Forsblom C, Liebkind R, Tatlisumak T, Thorn LM, FinnDiane Study Group (2021) Carotid intima-media thickness and arterial stiffness in relation to cerebral small vessel disease in neurologically asymptomatic individuals with type 1 diabetes. Acta Diabetol 58:929–937. https://doi.org/10.1007/s00592-021-01678-x
Polak JF, Backlund JYC, Cleary PA, Harrington AP, O’Leary DH, Lachin JM, DCCT/EDIC Research Group (2011) Progression of carotid artery intima-media thickness during 12 years in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes 60(2):607–613. https://doi.org/10.2337/db10-0296
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group (2003) Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348(23):2294–2303
Yamasaki Y, Kawamori R, Matsushima H, et al (1994) Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes 43(5):634–639
Larsen JR, Brekke M, Bergengen L, et al (2005) Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia 48(4):776–779. https://doi.org/10.1007/s00125-005-1700-z
Thorn LM, Forsblom C, Fagerudd J, et al (2005) Metabolic syndrome in type 1 diabetes. Diabetes Care 28(8):2019–2024
Warnick GR, Knopp RH, Fitzpatrick V, Branson L (1990) Estimating low-density lipoprotein cholesterol by the friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem 36(1):15–19
Levey AS, Inker LA, Coresh J (2014) GFR estimation: from physiology to public health. Am J Kidney Dis 63(5):820–834. https://doi.org/10.1053/j.ajkd.2013.12.006
Groop PH, Thomas MC, Moran JL, et al (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58(7):1651–1658. https://doi.org/10.2337/db08-1543
Wright LA, Hirsch IB (2017) Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabet Technol Ther 19(S2):S16–S26. https://doi.org/10.1089/dia.2017.0029
Wyman RA, Fraizer MC, Keevil JG, et al (2005) Ultrasound-detected carotid plaque as a screening tool for advanced subclinical atherosclerosis. Am Heart J 150(5):1081–1085. https://doi.org/10.1016/j.ahj.2005.01.010
Kozakova M, Morizzo C, Goncalves I, Natali A, Nilsson J, Palombo C (2019) Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol 18(1):61. https://doi.org/10.1186/s12933-019-0865-6
Kozakova M, Boutouyrie P, Morizzo C, et al (2018) Radiofrequency-based wall tracking for noninvasive assessment of local carotid pulse pressure: comparison with applanation tonometry and association with organ damage. J Hypertens 36(12):2362–2368. https://doi.org/10.1097/HJH.0000000000001837
van den Munckhof ICL, Jones H, Hopman MTE, et al (2018) Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol 41(5):698–704. https://doi.org/10.1002/clc.22934
Roumeliotis A, Roumeliotis S, Panagoutsos S, et al (2019) Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren Fail 41(1):131–138. https://doi.org/10.1080/0886022X.2019.1585372
Khawandanah J (2019) Double or hybrid diabetes: a systematic review on disease prevalence, characteristics and risk factors. Nutr Diabet. https://doi.org/10.1038/s41387-019-0101-1
Zhang L, Guo K, Lu J, et al (2016) Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness in type 1 Diabetic patients. Sci Rep 6:26805. https://doi.org/10.1038/srep26805
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25):2643–2653
Polak JF, Backlund JYC, Budoff M, Raskin P, Bebu I, Lachin JM, DCCT/EDIC Research Group (2021) Coronary artery disease events and carotid intima-media thickness in type 1 diabetes in the DCCT/EDIC cohort. J Am Heart Assoc 10(24):e022922. https://doi.org/10.1161/JAHA.121.022922
de Ferranti SD, de Boer IH, Fonseca V, et al (2014) Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American heart association and American diabetes association. Diabet Care 37(10):2843–2863. https://doi.org/10.2337/dc14-1720
Mahmud FH, Earing MG, Lee RA, Lteif AN, Driscoll DJ, Lerman A (2006) Altered endothelial function in asymptomatic male adolescents with type 1 diabetes. Congenit Heart Dis 1(3):98–103. https://doi.org/10.1111/j.1747-0803.2006.00015.x
Wink DA, Miranda KM, Espey MG, et al (2001) Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 3(2):203–213
Acknowledgements
The authors are indebted to the late Carol Forsblom (1964–2022), the international coordinator of the FinnDiane Study Group, for his considerable contribution throughout the years and for this specific study. The authors deeply acknowledge the technical assistance of Anna Sandelin, Jaana Tuomikangas, Kirsi Uljala, and Mira Korolainen from the Folkhälsan Research Center, Helsinki.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). The FinnDiane study was supported by grants from Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Liv och Hälsa Society, Medical Society of Finland (Finska Läkaresällskapet), Novo Nordisk Foundation (NNF23OC0082732), Sigrid Juselius Foundation, Finnish Foundation for Cardiovascular Research, and by State Funding for University-level Health Research (TYH 2023403). None of the funding bodies had any role in the study design; collection, analysis, or interpretation of data; writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
J.I., V.H., J.M., J.P., P–H.G., D.G., and L.M.T contributed to the study design and acquisition of data, as well as the interpretation of data. J.I., V.H., J.M., J.P., and D.G. had the main responsibility for analyzing data and writing the first draft of the paper. J J.I., V.H., J.M., J.P., P–H.G., D.G., and L.M.T critically revised the manuscript. P–H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
D.G. Lecture or Advisory Board Honoraria: Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Fresenius, GE Healthcare, Novo Nordisk, all outside the submitted work. J.M. Lecture Honoria Santen. P.-H.G. has received lecture honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Genzyme, Medscape, Merck Sharp & Dohme (MSD), Mundipharma, Novartis, Novo Nordisk, PeerVoice, Sanofi, SCIARC, and is an advisory board member of AbbVie, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Medscape, MSD, Mundipharma, Novartis, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District.
Consent to participation
Each participant signed a written informed consent before participation.
Consent for publication
Not applicable.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A complete list of the FinnDiane Study Group can be found in the supplementary material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inkeri, J., Harjutsalo, V., Martola, J. et al. No correlation between carotid intima-media thickness and long-term glycemic control in individuals with type 1 diabetes. Acta Diabetol 61, 441–449 (2024). https://doi.org/10.1007/s00592-023-02211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02211-y