Abstract
Aims
To explore the effect of liraglutide treatment on serum adropin and its relationship to the liver fat content in newly diagnosed patients with type 2 diabetes mellitus (T2DM) and metabolic dysfunction-associated fatty liver disease (MAFLD).
Methods
Serum adropin level and liver fat content were assessed in patients with T2DM and MAFLD (n = 22), along with healthy controls (n = 22). Afterward, the patients received liraglutide treatment for 12 weeks. Serum adropin levels were examined by a competitive enzyme-linked immunosorbent assay. Liver fat content was quantified via magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF).
Results
We found that patients with newly diagnosed T2DM and MAFLD had lower serum adropin levels [2.79 ± 0.47 vs. 3.27 ± 0.79 ng/mL, P < 0.05] and higher liver fat content [19.12 ± 9.46 vs. 4.67 ± 0.61%, P < 0.001], compared to healthy controls.
Following 12-week liraglutide treatment, serum adropin levels increased from 2.83(2.44, 3.24) to 3.65(3.20, 3.85) ng/mL (P < 0.001), and liver fat content decreased from 18.04(11.08, 27.65) to 7.74(6.42, 13.49) % (P < 0.001) in patients with T2DM and MAFLD. Furthermore, increases in serum adropin were strongly associated with decreases in liver fat content (β = − 5.933, P < 0.001), liver enzyme and glucolipid metabolism parameters.
Conclusion
The increase in serum adropin level following liraglutide treatment was strongly correlated with the reduction in liver fat content and glucolipid metabolism. Hence, adropin might be a potential marker for the beneficial effects of liraglutide on treating T2DM and MAFLD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly known as non-alcoholic fatty liver disease (NAFLD), has become a leading cause of chronic liver disease in the world [1,2,3]. MAFLD frequently coexists with type 2 diabetes (T2DM), which can drive adverse outcomes including diabetic macro- and micro-vascular complications, liver fibrosis, cirrhosis, and hepatic carcinoma [4]. At present, there has no approved pharmacotherapy for MAFLD. Liraglutide, a long-acting analog of glucagon-like peptide-1 (GLP-1), is effective in treating T2DM. Recent animal studies indicate that liraglutide reduces liver fat [5], hepatic steatosis [6], inflammation [7], and oxidative stress [8] in animal models of NAFLD. Clinical evidence shows that liraglutide ameliorates body weight [9], liver fat content (LFC) [10, 11], hepatic enzymes and improves liver histology [12] in patients with NAFLD. Hence, liraglutide is expected to be a promising agent of NAFLD, but the underlying mechanisms is still unknown.
Adropin, encoded by the energy homeostasis-associated (Enho) gene, is highly expressed in the liver and brain [13]. Accumulating evidence has illustrated that adropin has been linked to metabolism and energy homeostasis. Epidemiological studies have demonstrated that circulating adropin decreased in T2DM, coronary artery disease, polycystic ovary disease, and hypertension [14,15,16,17]. Our previous study has shown that serum adropin concentration reduced and inversely correlated with NAFLD activity score (NAS) in MAFLD patients with T2DM [18]. Kumar et al. found that adropin treatment alleviated hepatic steatosis and insulin resistance, reduced expression of hepatic lipogenic genes, and improved glucose homeostasis in diet-induced obese (DIO) mice [19]. These studies collectively show that adropin might be a potential biomarker for the pathophysiology of obesity-related metabolic diseases.
This study was designed to explore the effect of liraglutide treatment on serum adropin and its relationship to the liver fat content in newly diagnosed patients with T2DM and MAFLD. Given the invasiveness, high cost and complications of liver biopsy, we conducted magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), an emerging surrogate to diagnose and monitor the treatment response of NAFLD, to qualify liver fat content of all participants.
Methods
Participants
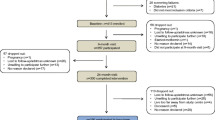
This 12-week prospective study was performed in the Department of Endocrinology in Beijing Chao Yang Hospital of Capital Medical University during the period from October 2018 to November 2019. We consecutively recruited 22 newly diagnosed patients with T2DM complicated by MAFLD, along with 22 age-, sex-matched healthy controls. Diagnosis of T2DM is based on the American Diabetes Association standard [20]. Patient selection met the following criteria: (1) diagnosed with T2DM within the previous 3 months and not use medications to treat diabetes; (2) age: 20–65 years; (3) body mass index (BMI) ≥ 24 kg/m2 [21]; (4) Hemoglobin A1c (HbA1c) ≥ 6.5% (48 mmol/mol); (5) liver fat content > 5.5% [22]. Participants were excluded for alcoholic hepatitis, drug-induced liver disease, autoimmune hepatitis, hepatocellular carcinoma, acute infectious diseases, acute myocardial infarction, hematological disorders, stroke, and any magnetic resonance imaging (MRI) contraindications.
Study design
Baseline
All participants underwent a clinical assessment including comprehensive medical history, anthropometric measurement, biochemical measurements, and MRI-PDFF. Eligible patients with T2DM complicated by MAFLD receive liraglutide for 12 weeks. Liraglutide was subcutaneously injected at a starting dose of 0.6 mg/d and increased by weekly to 1.8 mg/d. Meanwhile, all patients received the recommendations of appropriate management protocols by the current guidelines, including diabetes education, diet, and exercise.
Study visits
Patients attended regular follow-up every 4 weeks and took records of their symptoms and medication use. The adverse events were recorded all the time. During the treatment, 1(4.5%) developed mild upper gastrointestinal upset and 1(4.5%) developed diarrhea; patients improved after symptomatic treatment. After completion of 12-week liraglutide administration, clinical assessment and fasting blood samples were assessed again.
Ethics
Ethical approval complying with the Helsinki Declaration was given by the Ethics Committee of Beijing Chao-yang Hospital, Capital Medical University. All participants voluntarily signed written informed consent statements prior to study initiation.
Clinical assessment
Anthropometric characteristics (height, weight) were measured by a professional. The body mass index (BMI) was calculated as BMI = kg/m2. Fasting blood samples were undertaken and stored at − 80 °C. Blood samples were assayed in the central laboratory of the hospital for the following indices: lipid profile[total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG)]; fasting blood glucose (FBG); fasting insulin (FINS); HbA1c; liver function test [alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), total bile acids (TBA)]; and free fatty acid (FFA). Homoeostasis model assessment of insulin resistance (HOMA-IR) = FPG (mmol/L) × FINS (mU/L)/22.5; Homoeostasis model assessment of β-cell function (HOMA-β) = 20 × FINS (mU/L)/FPG (mmol/L)—3.5 [23].
Serum adropin levels were quantified by a competitive human adropin enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals, Burlingame, CA, USA). The kit was used according to the manufacturers’ protocols with a sensitivity of 0.3 ng/mL. The serum adropin standards ranged from 0.3 and 8.2 ng/mL. The coefficient of variation were 10% intra-assay and 15% inter-assay.
MRI-PDFF protocols
MRI examination
MRI-PDFF, a quantitative, accurate, and non-invasive imaging-based biomarker, enables us to measure the liver lipid content reproducibly [24,25,26,27]. In this study, imaging assessments were performed at baseline and weeks 12 by an experienced MRI technologist. An upper-abdominal MRI scan for each participant was performed under 12 h fasting conditions using an identical equipment set-up throughout the study. All spectra were acquired on a whole body 3-Tesla MRI scanner (Siemens Medical Solutions, Erlangen, Germany). MRI scanner protocol are shown below: (1) the localizer images were set initially; (2) a T1 volumetric interpolated breath-hold examination (VIBE) Dixon sequence was adopted with parameters: echo time (TE) 1 = 1.23 ms; TE2 = 2.46 ms; repetition time (TR) = 3.97 ms; bandwidth (BW) 1 = 1040 Hz/Px; BW2 = 1040 Hz/Px; flip angle = 9°; slice thickness = 3.0 mm.
MRI postprocessing
The MR images were transmitted through an image processing workstation to a Siemens Syngo. and to a radiologist for analysis. Fat-fraction map images were calculated from the in-phase and fat-phase raw data. The hepatic proton density fat fraction (PDFF) was measured with regions of interest (ROIs) in fat-fraction map image using the MITK 3M3 software (downloadable at http://www.mitk.org/). Tracing the liver boundaries were determined manually, excluding portal vein, inferior vena cava, bile ducts, focal hepatic lesions, and imaging artifacts. All images and data were supervised by a blinded senior radiologist.
Statistical analysis
The data were expressed as mean ± SD or median (IQR). Statistical analyses were conducted by SPSS 23.0 (IBM Corporation, NY, USA). The characteristics at baseline were compared using the independent sample Student’s t-test (normally distributed data) or Mann–Whitney U test (skewed data). Within-group comparations (pre- and post-treatment) were done with paired Student’s t-test or nonparametric Wilcoxon test to assess the change of clinical parameters from baseline to the end in patients with T2DM and MAFLD. The Pearson or Spearman analyses coefficients and p-values were calculated among serum adropin levels, liver fat content and metabolic parameters at baseline. Moreover, we adopted linear mixed-effects models to evaluate the longitudinal relationship between serum adropin levels and liver fat content during 12-week treatment, which was performed by STATA 13.0 (STATA, College Station, TX). P < 0.05 were considered statistically significant.
Results
Baseline characteristics of participants
Baseline clinical characteristics of all subjects were presented in Table 1. The distribution of age and sex was similar across the two groups. Compared to the matched controls, patients with T2DM and MAFLD had higher BMI, TC, TG, FBG, FINS, HbA1c, HOMA-IR, AST, ALT, GGT, TBA, and FFA, and lower HDL-C and HOMA-β (all P < 0.05). Notably, the patients in pre-treatment group exhibited lower serum adropin levels [2.79 ± 0.47 vs. 3.27 ± 0.79 ng/mL, P < 0.05] and higher liver fat content [19.12 ± 9.46 vs. 4.67 ± 0.61, P < 0.001] than the control group (Table1, Fig. 2).
Correlations of serum adropin levels and clinical parameters
In T2DM group, serum adropin levels correlated inversely with BMI, LDL-C, TG, HbA1c, TBA, FFA and liver fat content (BMI: r = − 0.328, LDL-C: r = − 0.326, TG: r = − 0.346, HbA1c: r = − 0.298, TBA: r = − 0.537, FFA: r = − 0.316, liver fat content: r = − 0.310; all P < 0.05), whereas correlated positively with HDL-C (HDL-C: r = 0.616; P < 0.01), as described in Table 2. The control groups had only one parameter, serum TBA (r = -0.572, P < 0.01), negatively associated with serum adropin level.
Effect of liraglutide on serum adropin levels, liver fat content, and metabolic parameters
Table 3 exhibited clinical and biological changes in patients with T2DM and MAFLD according to 12 weeks of treatment with liraglutide. Changes between pre- and post-treatment have shown significant decreases in BMI, TC, TG, FBG, HbA1c, HOMA-IR, AST, ALT, GGT, and FFA levels, as well as considerable increase in HOMA-β level (TC, HOMA-IR: P < 0.05; other indices: P < 0.01).
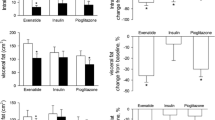
In addition, serum adropin levels elevated from 2.83(2.44, 3.24) to 3.65(3.20, 3.85) (P < 0.001, Fig. 1A and Table 3) and liver fat content reduced from 18.04(11.08, 27.65) to 7.74(6.42, 13.49) (P < 0.001, Figs. 1B, 2 and Table 3) following liraglutide treatment.
MRI-PDFF assessment among the control, pre-treatment, and post-treatment groups, respectively. The figure shows a slice from each subject and a yellow circle marked the liver fat content. The control subject is a 33 years old man who has a 4.93% liver fat content. Another sex-, age-matched patient with T2DM and MAFLD shows a decrease in PDFF from 29.87 to 6.87% following liraglutide treatment
Correlations between elevated serum adropin and changes in liver fat content and metabolic parameters
In T2DM group, longitudinal changes by treatment were assessed by linear mixed effects models. Two-class linear mixed models were fitted, and the data were summarized in Table 4. In model 1, without adjustment, higher levels of serum adropin were related to lower BMI (β = − 2.247, P = 0.013), HbA1c (β = − 1.575, P < 0.001), FBG (β = − 1.653, P = 0.002), AST (β = − 7.807, P = 0.014), FFA (β = − 0.087, P = 0.047). In model 2, adjusted for sex and age, the results of BMI, HbA1c, FBG, and AST remained similar to model 1 except for FFA (β = − 0.084, P = 0.058) and ALT (β = − 12.611, P = 0.018).
Moreover, we observed that increases in serum adropin were strongly associated with decreases in liver fat content during 12-week liraglutide treatment even after adjusting for sex and age (β = − 5.933, P < 0.001).
Discussion
In the present study, we confirmed that patients with T2DM and MAFLD had lower serum adropin levels and higher liver fat content than healthy controls. Liraglutide significantly elevated serum adropin levels, reduced liver fat content, and improved liver enzyme and other metabolic parameters. Notably, we provide the first evidence that the increased serum adropin level was strongly correlated with the decreased liver fat content following liraglutide treatment in patients with T2DM complicated by MAFLD.
Adropin, a secreted peptide, was first identified in the liver of obese mouse models in 2008 [19]. Thereafter, numerous studies have reported that adropin may be a potential regulator governing energy and metabolism homeostasis. Animal studies have shown that lower plasma adropin concentrations were observed in high-fat diet mice [28] or high-fructose diet rhesus macaques [29]. Similarly, an inverse correlation between circulating adropin concentrations and BMI was confirmed by human studies [17, 30], indicating that a low level of adropin is a hallmark of obesity.
Consistent with prior studies [17, 30, 31], our findings also suggested that serum adropin was negatively associated with BMI, LDL-C, TG, HbA1c and FFA while positively associated with HDL-C in patients with T2DM and MAFLD. Chen et al. reported that adropin-deficiency mice exhibited severe glucose homeostasis impairment and worse metabolism disorder [32]. Furthermore, the administration of synthetic adropin promotes glycogen synthesis, attenuates glucose production, and improves insulin sensitivity by raising IRS1/2-Akt phosphorylation and lowering the FoxO1 transcript in mouse models of diet-induced obesity [33]. Jasaszwili et al. [34] reported that adropin impaired preadipocyte differentiation, reduced the fat volume of brown adipose tissue, and improved the outflow of glycerol and FFA, which indicated that adropin involved in the modulation of lipid metabolism. Interestingly, we also found low serum adropin were related to high serum total bile acids, which provides a novel perspective on the role of adropin. Taken together, these results showed that low levels of adropin, which is correlated with impaired glucolipid metabolism and exacerbated insulin resistance, might be a pathogenetic factor involved in T2DM.
Multiple studies have proven that high levels of circulating adropin contributed to improved glucose tolerance, reduced insulin resistance, and ameliorated hyperlipidemia [28, 35, 36]. However, due to the high costs and time-consuming of developing new drugs, it is more effective to explore the potential of current drugs. In obese Wistar rats with T2DM, exogenous injection of adropin resulted in reduced blood glucose level, improved insulin sensitivity, ameliorated hyperlipidemia, and inhibited levels of inflammatory cytokines [37]. A recent human study of 15 obese male T2DM patients demonstrated that plasma adropin concentration increased significantly after treatment with liraglutide and metformin for three months [38]. Similarly, our results confirmed that liraglutide elevated serum levels of adropin. Simultaneously, the increased adropin was associated with weight loss, improved glucolipid metabolism, and alleviation of insulin resistance. Collectively, the results above indicated that adropin upregulation might be a novel mechanism for the beneficial effects of liraglutide in patients with T2DM.
Of note, in the current study, liver fat content was qualified by MRI-PDFF, which is considered to be a novel biomarker of MAFLD [26, 37]. In accordance with other studies [10, 12], our results demonstrated that liraglutide treatment for 12 weeks reduced liver fat content and liver enzyme, which indicated the beneficial effects of liraglutide in MAFLD. There are several possible mechanisms. First, Wu et al. [39] reported that liraglutide could ameliorate hepatic lipid accumulation via promoting reversal of cholesterol transport in diet-induced obese db/db mice. Second, Liraglutide activated autophagic flux and attenuated hepatic steatosis through the TFEB-mediated autophagy-lysosomal pathway [40]. Finally, HFD-fed genetically engineered mouse model demonstrated that liraglutide ameliorated lipid-induced hepatic steatosis though the HIF-2α/PPARα pathway [41]. Conversely, an earlier small study did not indicate an alleviation of LFC following 12-week liraglutide treatment [11]. The discrepancy might be due to differences in participants, disease duration, ongoing treatments and diagnostic techniques.
Interestingly, an inverse correlation between serum adropin levels and liver fat content was observed in patients with T2DM and MAFLD before and after liraglutide treatment. Chen et al. reported that serum adropin reduced in the non-alcoholic steatohepatitis mice and liver damage (such as hepatic steatosis and fibrosis) exacerbated in the adropin deficient mice fed with either western diet or methionine-choline deficient diet [42]. In fact, clinical studies revealed significantly low circulating adropin levels in patients with NAFLD, which indicated that adropin may involve in the pathophysiology of NAFLD [43, 44]. Moreover, animal studies revealed that adropin decreased the expression of hepatic lipogenic genes and adipose tissue PPARγ gene in DIO mouse model [19, 33]. Adropin also resists oxidative stress by upregulating Nrf2, and attenuating liver injury of NASH mice [42]. In our study, the longitudinal analysis showed that an elevation of serum adropin levels was related to the decline of liver fat content and hepatic enzymes during liraglutide treatment. This result may suggest that liraglutide increased serum adropin levels, exerting significant effects on improving liver fat deposition. Taken together, these results demonstrate that adropin might be involved in the beneficial effect of liraglutide on liver steatosis in patients with MAFLD.
Our study has some limitations. First, liver fat content was quantified via MRI-PDFF rather than the gold standard liver biopsy. Given the exploratory nature of our study, performing liver biopsies even in a research setting of T2DM and MAFLD comorbidity would hardly accepted by patients who are newly diagnosed. Second, owing to the small sample size and non-randomization design, bias may have been presented; hence, larger randomized trials are warranted to validate our findings. Furthermore, further animal and cell-based experiments are required to explore the underlying molecular mechanism of liraglutide and other GLP-1 receptor agonists (dulaglutide, semaglutide) on adropin and liver fat content. Nonetheless, our findings reflected the real-world clinical settings and exhibited adequate reliability with sophisticated statistical methods.
In conclusion, our study indicated that liraglutide significantly elevated serum adropin levels in patients with T2DM and MAFLD. The increase in serum adropin level following liraglutide treatment was strongly correlated with the improvement of liver fat content and glucolipid metabolism. Hence, adropin might be a potential marker involved in the beneficial effects of liraglutide on treating T2DM and MAFLD.
References
Younossi Z, Tacke F, Arrese M et al (2019) Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69(6):2672–2682. https://doi.org/10.1002/hep.30251
Sarin SK, Kumar M, Eslam M et al (2020) Liver diseases in the Asia-Pacific region: a Lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol 5(2):167–228. https://doi.org/10.1016/S2468-1253(19)30342-5
Eslam M, Sanyal AJ, George J (2020) MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158(7):1999–2014. https://doi.org/10.1053/j.gastro.2019.11.312
Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW (2016) Non-alcoholic fatty liver disease and diabetes. Metabolism 65(8):1096–1108. https://doi.org/10.1016/j.metabol.2016.01.001
Tolbol KS, Kristiansen MN, Hansen HH et al (2018) Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J Gastroenterol 24(2):179–194. https://doi.org/10.3748/wjg.v24.i2.179
Tan Y, Zhen Q, Ding X et al (2022) Association between use of liraglutide and liver fibrosis in patients with type 2 diabetes. Front Endocrinol. https://doi.org/10.3389/fendo.2022.935180
Duparc T, Briand F, Trenteseaux C et al (2019) Liraglutide improves hepatic steatosis and metabolic dysfunctions in a 3-week dietary mouse model of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 317(4):G508–G517. https://doi.org/10.1152/ajpgi.00139.2019
Tong W, Ju L, Qiu M et al (2016) Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res 46(9):933–943. https://doi.org/10.1111/hepr.12634
Knudsen LB, Lau J (2019) The discovery and development of liraglutide and semaglutide. Front Endocrinol. https://doi.org/10.3389/fendo.2019.00155
Petit JM, Cercueil JP, Loffroy R et al (2017) Effect of Liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the Lira-NAFLD study. J Clin Endocrinol Metab 102(2):407–415. https://doi.org/10.1210/jc.2016-2775
Tang A, Rabasa-Lhoret R, Castel H et al (2015) Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes_ a randomized trial. Diabetes Care 38(7):1339–1346. https://doi.org/10.2337/dc14-2548/-/DC1
Armstrong MJ, Gaunt P, Aithal GP et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387(10019):679–690. https://doi.org/10.1016/S0140-6736(15)00803-X
Jasaszwili M, Billert M, Strowski MZ, Nowak KW, Skrzypski M (2020) Adropin as a fat-burning hormone with multiple functions-review of a decade of research. Molecules. https://doi.org/10.3390/molecules25030549
Gulen B, Eken C, Kucukdagli OT et al (2016) Adropin levels and target organ damage secondary to high blood pressure in the ED. Am J Emerg Med 34(11):2061–2064. https://doi.org/10.1016/j.ajem.2016.04.014
Zheng J, Liu M, Chen L et al (2019) Association between serum adropin level and coronary artery disease: a systematic review and meta-analysis. Cardiovasc Diagn Ther 9(1):1–7. https://doi.org/10.21037/cdt.2018.07.09
Ye Z, Zhang C, Zhao Y (2021) Potential effects of adropin on systemic metabolic and hormonal abnormalities in polycystic ovary syndrome. Reprod Biomed Online 42(5):1007–1014. https://doi.org/10.1016/j.rbmo.2021.01.020
Zang H, Jiang F, Cheng X, Xu H, Hu X (2018) Serum adropin levels are decreased in Chinese type 2 diabetic patients and negatively correlated with body mass index. Endocr J 65(7):685–691. https://doi.org/10.1507/endocrj.EJ18-0060
Li N, Xie G, Zhou B et al (2021) Serum adropin as a potential biomarker for predicting the development of type 2 diabetes mellitus in individuals with metabolic dysfunction-associated fatty liver disease. Front Physiol. https://doi.org/10.3389/fphys.2021.696163
Kumar KG, Trevaskis JL, Lam DD et al (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 8(6):468–481. https://doi.org/10.1016/j.cmet.2008.10.011
American Diabetes, A (2013) Standards of medical care in diabetes–2013. Diabetes Care 36(Suppl 1):S11-66. https://doi.org/10.2337/dc13-S011
CSo, E (2011) Chinese expert consensus for the prevention and treatment of obesity in adults. Chin J Endocrinol Metab. https://doi.org/10.3760/cma.j.issn.1000-6699.2011.09.003
Szczepaniak LS, Nurenberg P, Leonard D et al (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288(2):E462–E468. https://doi.org/10.1152/ajpendo.00064.2004
Bermúdez V, Cano R, Cano C et al (2008) Homeostasis model assessment (HOMA) as surrogate insulinization criteria in patients with type 2 diabetes. Am J Ther 15(4):409–416. https://doi.org/10.1097/MJT.0b013e318160b909
Reeder SB (2013) Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology 58(6):1877–1880. https://doi.org/10.1002/hep.26543
Ajmera V, Loomba R (2021) Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. https://doi.org/10.1016/j.molmet.2021.101167
Caussy C, Reeder SB, Sirlin CB, Loomba R (2018) Noninvasive, Quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 68(2):763–772. https://doi.org/10.1002/hep.29797
Noureddin M, Lam J, Peterson MR et al (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58(6):1930–1940. https://doi.org/10.1002/hep.26455
Ganesh Kumar K, Zhang J, Gao S et al (2012) Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity 20(7):1394–1402. https://doi.org/10.1038/oby.2012.31
Butler AA, Zhang J, Price CA et al (2019) Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J Biol Chem 294(25):9706–9719. https://doi.org/10.1074/jbc.RA119.007528
Butler AA, Tam CS, Stanhope KL et al (2012) Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab 97(10):3783–3791. https://doi.org/10.1210/jc.2012-2194
Yuan X, Chen R, Ouyang Q et al (2020) Novel associations of serum adropin and lipopolysaccharide-binding protein versus lipid profiles in childhood obesity. J Pediatr Endocrinol Metab 33(2):265–270. https://doi.org/10.1515/jpem-2019-0329
Chen S, Zeng K, Liu QC et al (2017) Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis 8(8):e3008. https://doi.org/10.1038/cddis.2017.362
Gao S, Ghoshal S, Zhang L et al (2019) The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J Biol Chem 294(36):13366–13377. https://doi.org/10.1074/jbc.RA119.008967
Jasaszwili M, Wojciechowicz T, Strowski MZ, Nowak KW, Skrzypski M (2020) Adropin stimulates proliferation but suppresses differentiation in rat primary brown preadipocytes. Arch Biochem Biophys. https://doi.org/10.1016/j.abb.2020.108536
Chen X, Chen S, Shen T et al (2020) Adropin regulates hepatic glucose production via PP2A/AMPK pathway in insulin-resistant hepatocytes. FASEB J 34(8):10056–10072. https://doi.org/10.1096/fj.202000115RR
Gao S, McMillan RP, Jacas J et al (2014) Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 63(10):3242–3252. https://doi.org/10.2337/db14-0388
Akcilar R, Kocak FE, Simsek H et al (2016) Antidiabetic and hypolipidemic effects of adropinin streoptozotocin-induced type 2 diabetic rats. Bratisl Lek Listy 117(2):100–105. https://doi.org/10.4149/bll_2016_020
Ticinovic Kurir T, Milicevic T, Novak A, Vilovic M, Bozic J (2020) Adropin—potential link in cardiovascular protection for obese male type 2 diabetes mellitus patients treated with liraglutide. Acta Clin Croat 59(2):344–350. https://doi.org/10.20471/acc.2020.59.02.19
Wu YR, Shi XY, Ma CY, Zhang Y, Xu RX, Li JJ (2019) Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc Diabetol 18(1):146. https://doi.org/10.1186/s12933-019-0954-6
Fang Y, Ji L, Zhu C et al (2020) Liraglutide alleviates hepatic steatosis by activating the TFEB-regulated autophagy-lysosomal pathway. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.602574
Wang H, Wang L, Li Y et al (2021) The HIF-2alpha/PPARalpha pathway is essential for liraglutide-alleviated, lipid-induced hepatic steatosis. Biomed Pharmacother 140:111778. https://doi.org/10.1016/j.biopha.2021.111778
Chen X, Xue H, Fang W et al (2019) Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. https://doi.org/10.1016/j.redox.2018.101068
Chen X, Sun X, Shen T et al (2020) Lower adropin expression is associated with oxidative stress and severity of nonalcoholic fatty liver disease. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2020.08.005
Kutlu O, Altun O, Dikker O et al (2019) Serum adropin levels are reduced in adult patients with nonalcoholic fatty liver disease. Med Princ Pract 28(5):463–469. https://doi.org/10.1159/000500106
Funding
This work was supported by grants from Beijing Natural Science Foundation Z20019 to J.L.
Author information
Authors and Affiliations
Contributions
GW and JL conceived and designed the experiments; XJW, XNC, and XYD recruited patients and collected patient samples; LZ and QW performed the experiments; XYL and TJ performed MRI examination and postprocessing; LZ analyzed the patient data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
Informed written consent was received from all the participants prior to the study initiation. Approval was obtained from the ethics committee of Beijing Chao-yang Hospital, Capital Medical University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
All participants voluntarily signed written informed consent statements prior to study initiation.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Wu, X., Li, X. et al. Longitudinal changes in serum adropin levels and liver fat content during liraglutide treatment in newly diagnosed patients with type 2 diabetes mellitus and metabolic dysfunction-associated fatty liver disease. Acta Diabetol 60, 971–979 (2023). https://doi.org/10.1007/s00592-023-02082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02082-3