Abstract
Aims
To evaluate the impact of adding a glucagon-like peptide-1 receptor agonist (GLP-1 RA) in people with type 2 diabetes (T2D) in basal-bolus (BB) insulin regimen, on insulin requirement, HbA1c, weight loss up to 24 months.
Methods
Data on subjects with T2D on BB who initiated a GLP-1 RA have been retrospectively collected. HbA1c, body weight, and insulin dose were recorded at baseline, 6, 12, and 24 months after initiation of GLP-1 RA therapy. A linear mixed model for repeated measures was used to evaluate the changes in HbA1c, body weight, and insulin requirement over time.
Results
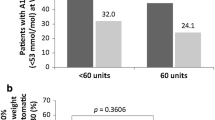
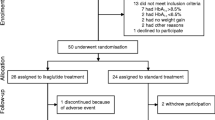
We included 156 subjects (63.5% males; age 62 ± 11 years, HbA1c 70 ± 22.0 mmol/mol; 8.6 ± 4.2%). Compared to baseline, HbA1c and body weight were significantly lower at 6 months after introducing a GLP-1RA and remained stable up to 24 months (all p < 0.0001 vs. baseline). At 24 months, 81% of subjects discontinued prandial insulin, while 38.6% discontinued basal insulin as well. Insulin requirement at baseline (aOR 0.144; 95% CI, 0.046–0.456; P = 0.001) was the only significant predictor of prandial insulin discontinuation.
Conclusions
Replacing prandial insulin with GLP-1 RA is a valuable strategy to simplify the BB insulin regimen while improving glycaemic control and promoting weight loss in subjects with T2D.



Similar content being viewed by others
Data availability
The dataset generated during and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Care D, Suppl SS (2022) 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. Diabetes Care 45:S125–S143. https://doi.org/10.2337/dc22-S009
Basu S, Yudkin JS, Kehlenbrink S, Davies JI, Wild SH, Lipska KJ et al (2019) Estimation of globalinsulin use for type 2 diabetes, 2018–30: a microsimulation analysis. Lancet Diabetes Endocrinol 7:25–33. https://doi.org/10.1016/S2213-8587(18)30303-6
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G et al (2018) Management of hyperglycaemia in type 2 diabetes a consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia 61(12):2461–2498. https://doi.org/10.2337/dci18-0033
Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C et al (2020) Erratum: 2019 updateto: Management of hyperglycemia in type 2 diabetes, 2018 A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 43(2):487–493. https://doi.org/10.2337/dc20-er07
Diamant M, Nauck MA, Shaginian R, Malone JK, Cleall S, Reaney M et al (2014) Glucagon-likepeptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 37:2763–2773. https://doi.org/10.2337/dc14-0876
Eng C, Kramer CK, Zinman B, Retnakaran R (2014) Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384:2228–2234. https://doi.org/10.1016/S01406736(14)61335-0
Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D (2017) Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic reviewand meta-analysis of randomized controlled trials. Diabetes Care 40:614–624. https://doi.org/10.2337/dc16-1957
Aroda VR, Rosenstock J, Wysham C, Unger J, Bellido D, González-Gálvez G et al (2016) Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 39:1972–1980. https://doi.org/10.2337/dc16-1495
Lingvay I, Manghi FP, García-Hernández P, Norwood P, Lehmann L, Tarp-Johansen MJ et al (2016) Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levelsin patients with uncontrolled type 2 diabetes: the DUAL v randomized clinical trial. JAMA J Am Med Assoc 315:898–907. https://doi.org/10.1001/jama.2016.1252
Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F (2019) GLP-1 receptor agonist added toinsulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review andmeta-analysis. Diabetes Metab Res Rev 35:e3082. https://doi.org/10.1002/dmrr.3082
Bonora BM, Rigato M, Frison V, D’Ambrosio M, Tadiotto F, Lapolla A et al (2021) Deintensification of basal-bolus insulin after initiation of GLP-1RA in patients with type 2 diabetes under routine care. Diabetes Res Clin Pract 173:108686. https://doi.org/10.1016/j.diabres.2021.108686
Rosenstock J, Nino A, Soffer J, Erskine L, Acusta A, Dole J et al (2020) Impact of a weeklyglucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabetes Care 43:2509–2518. https://doi.org/10.2337/DC19-2316
Wilcox T, De Block C, Schwartzbard AZ, Newman JD (2020) Diabetic agents, from metformin tosglt2 inhibitors and glp1 receptor agonists: JACC focus seminar. J Am Coll Cardiol 75:1956–1974. https://doi.org/10.1016/j.jacc.2020.02.056
Giugliano D, Longo M, Caruso P, Di Fraia R, Scappaticcio L, Gicchino M et al (2021) Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a glp-1ra or to basal insulin plus an sglt2 inhibitor: beyond, a randomized, pragmatic trial. Diabetes Care 44:1353–1360. https://doi.org/10.2337/DC20-2623
Price H, Blüher M, Prager R, Phan TM, Thorsted BL, Schultes B (2018) Use and effectiveness of afixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population withtype 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab 20:954–962. https://doi.org/10.1111/dom.13182
Hopton OM, Waterbury NV, Egge JA, Lund BC (2022) Clinical impact of GLP-1 receptor agonistsin veterans receiving basal-bolus insulin. Pharmacotherapy 42:45–52. https://doi.org/10.1002/phar.2648
Yoon NM, Cavaghan MK, Brunelle RL, Roach P (2009) Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 31:1511–1523. https://doi.org/10.1016/j.clinthera.2009.07.021
Taybani Z, Bótyik B, Katkó M, Gyimesi A, Várkonyi T (2019) Simplifying complex insulin regimenswhile preserving good glycemic control in type 2 diabetes. Diabetes Ther 10:1869–1878. https://doi.org/10.1007/S13300-019-0673-8
Nauck MA, Quast DR, Wefers J, Meier JJ (2021) GLP-1 receptor agonists in the treatment of type 2diabetes–state-of-the-art. Mol Metab 46:101102. https://doi.org/10.1016/j.molmet.2020.101102
Jones AG, McDonald TJ, Shields BM, Hill AV, Hyde CJ, Knight BA et al (2016) Markers of β-cellfailure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care 39:250–257. https://doi.org/10.2337/dc15-0258
Del Prato S, Frias JP, Blonde L, Aroda VR, Shehadeh N, Saremi A et al (2020) Impact of disease duration and β-cell reserve on the efficacy of switching to iGlarLixi in adults with type 2 diabeteson glucagon-like peptide-1 receptor agonist therapy: exploratory analyses from the LixiLan-G trial. Diabetes Obes Metab 22(9):1567–1576. https://doi.org/10.1111/dom.14068
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PF, FN and CB contributed to the conception of the study, researched data and wrote the manuscript. FC, AC, MG and SDP contributed to the acquisition and interpretation of clinical data and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
PF, FN, FC, AC reports no duality of interest. MG reports consulting fees from Lilly. SDP declares grants from AstraZeneca and Boehringer Ingelheim, consulting fees from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharpe and Dohme, Novartis Pharmaceuticals, Novo Nordisk and Sanofi, and honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharpe and Dohme, Novartis Pharmaceuticals, Novo Nordisk and Sanofi. CB reports consulting fees and grants from Lilly, Novo Nordisk, Roche Diagnostics.
Ethics approval and informed consent
The study protocol was approved by the local Ethics Committee of the University of Pisa and conducted in accordance with the Declaration of Helsinki. Since data were anonymous and used in aggregated form no subjects’ informed consent was needed according to local ethic committee indications.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Falcetta, P., Nicolì, F., Citro, F. et al. De-intensification of basal-bolus insulin regimen after initiation of a GLP-1 RA improves glycaemic control and promotes weight loss in subjects with type 2 diabetes. Acta Diabetol 60, 53–60 (2023). https://doi.org/10.1007/s00592-022-01974-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01974-0