Abstract
Aims
Disturbances in circadian rhythms may promote cardiometabolic disorders in rotating night shift workers (r-NSWs). We hypothesized that timed light therapy might reverse disrupted circadian rhythms and glucose intolerance observed among r-NSWs).
Methods
R-NSWs were randomly assigned to a protocol that included 12 weeks on followed by 12 weeks off light therapy (n = 13; 6 men; mean age, 39.5 ± 7.3 years) or a no-treatment control group (n = 9; 3 men; mean age 41.7 ± 6.3 years). Experimental and control participants underwent identical metabolic evaluations that included anthropometric, metabolic (including oral glucose tolerance tests), lipid, and inflammation-associated parameters together with an assessment of sleep quality and expression of circadian transcription factors REV-ERBα and BMAL1 in peripheral blood mononuclear cells (PBMCs) at baseline, 12 weeks, and 24 weeks of the protocol.
Results
Twelve weeks of warm white-light exposure (10,000 lx at 35 cm for 30 min per day) had no impact on sleep, metabolic, or inflammation-associated parameters among r-NSWs in the experimental group. However, our findings revealed significant decreases in REV-ERBα gene expression (p = 0.048) and increases in the REV-ERBα/BMAL1 ratio (p = 0.040) compared to baseline in PBMCs isolated from this cohort. Diminished expression of REV-ERBα persisted, although the REV-ERBα/BMAL1 ratio returned to baseline levels after the subsequent 12-day wash-out period.
Conclusions
Our results revealed that intermittent light therapy had no impact on inflammatory parameters or glucose tolerance in a defined cohort of r-NSWs. However, significant changes in the expression of circadian clock genes were detected in PBMCs of these subjects undergoing light therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many workers in Western societies perform night shift work. One recent review reported that as many as 20% of the working population in Europe is engaged in some type of shift work schedule [1]. Among those employed in the health care sector, this percentage increases to ~ 45% [1, 2]. Biological rhythms may not be well-tuned to or capable of rapid adaptation to changes in these environmental cycles and reduced sleep; frequent time shifts may predispose workers to poor metabolic health [3]. Results from recent epidemiological studies have suggested that rotating night shift work is associated with a higher risk of developing type 2 diabetes (T2D), increased inflammation, and cardiovascular disease [4,5,6,7]. Even when the diet is controlled, night-shift workers (NSWs) exhibit poorer metabolic health than those whose work takes place during daytime hours [8]. As but one example, results from the Nurse Health Study II revealed that participants who reported sleep difficulties when working on rotating night shifts had an increased risk of developing T2D [9]. Our group recently reported subclinical abnormalities in HbA1c and changes in expression of circadian clock genes among current NSWs compared to former NSWs and individuals who work during daylight hours only [10]. Prolonged exposure to artificial light also contributes to the disruption of the internal clock. Several studies have revealed that exposure to artificial light at night promoted significant metabolic disturbances in experimental animal models [11, 12]. Furthermore, the results of one recent study revealed that daylight was more effective than exposure to artificial light at night (LAN) for suppression of melatonin excretion in urine. These results suggest that there may be other, as yet unidentified biomarkers that might be used to identify subjects who are sensitive to the negative effects of rotating shift work and LAN [13]. Thus, we performed a small hypothesis-generating study aimed at analyzing the impact of warm white light exposure on the expression of circadian clock genes and several characterized inflammatory and metabolic biomarkers in a cohort of rotating NSWs.
Methods
Light therapy protocol
A randomized open-label clinical trial that included a 12-week session of light intervention followed by a 12-week off-protocol period was performed at Policlinico Tor Vergata between 2013 and 2015 as part of the EuRhythDia project. The EuRhythDia consortium investigated potential disruptions of the circadian clock in rotating NSWs and characterized the impact of light therapy. The overall goal was to determine whether this intervention might improve insulin sensitivity and limit inflammation in individuals belonging to high-risk groups. Participants were active rotating NSWs working a shift schedule that included four to as many as seven 12 h overnight shifts nights per month followed by two days off for a minimum of two years. Exclusion criteria included a diagnosis of diabetes, liver disease, renal insufficiency, heart failure, coagulopathy, or any other severe systemic disease. Subjects were also excluded if they had a history of cancer in any form, positive blood tests for human immunodeficiency virus, hepatitis B, and/or hepatitis C, and/or if they had taken melatonin supplements within four weeks before beginning the study. In women, the study was initiated during the early follicular phase of the menstrual cycle. Participants provided written consent after receiving detailed information about the study protocol. The Tor Vergata University Ethics Board approved this study (decision #EU-FP3:125/13). The investigations reported in this study were carried out following the principles of the Declaration of Helsinki (revised in 2000).
Twenty-eight (n = 28) subjects were recruited and randomized on a one-to-one basis to the light intervention therapy (n = 14) or control groups (n = 14). Upon commencing the study and after the first and second 12-week periods as described, anthropometric data and 12-h fasting blood samples were collected from all study participants.
Plasma glucose levels were measured in vitro by an enzymatic test (automated chemistry clinical analyzer, Roche). Serum insulin levels were determined using an electrochemiluminescence assay (Eclia, Roche). A 75 g oral glucose tolerance test (OGTT) with sampling at 0, 30, 60, 90, and 120 min for plasma glucose and serum insulin levels was performed for each subject.
Real-time (RT)-polymerase chain reaction (PCR)
Approximately 20 ml samples of whole blood were drawn from each subject at each timepoint. Approximately eight ml of blood drawn into a Vacutainer with sodium citrate anticoagulant (Becton–Dickinson) was used to isolate peripheral blood mononuclear cells (PBMCs) as previously described [10, 14]. Single-stranded cDNA was synthesized from 2 μg of total RNA using a High-Capacity cDNA Archive Kit as per the standard protocol (Applied Biosystems, Foster City, CA, USA). Fifty nanogram samples of cDNA were amplified by RT-PCR to assess the expression of circadian clock (REV-ERBα and BMAL1) and inflammatory (IL-1β and IFN-γ) genes using an ABI PRISM 7500 System. Results were normalized to 18S rRNA as the endogenous control.
Assessment of sleep quality
Perception of sleep during study protocol was determined using the Pittsburgh Sleep Quality Index (PSQI), which is a validated scale that can be used to identify elements of sleep over a previous 30-day period [10]. Briefly, the questionnaire explores seven different components of sleep, including sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, daytime dysfunction, and subjective sleep quality. The sum of scores of these seven components provides a global PSQI score that ranges from 0 to 21 points, with a score of 5 or greater indicative of poor sleep quality. We used this information to dichotomize the scores [good sleepers (score < 5) vs poor sleepers (score ≥ 5)] in our analysis.
Light therapy
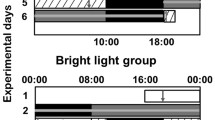
Subjects randomized to the light therapy group maintained their standard habits (i.e., diet, exercise activity) and sleep–wake patterns on non-night shift days as they had before the study period. Participants were exposed to bright light (10,000 lx at a distance of 35 cm for 30 min) every morning at home between 06:00 and 09:00 h except on those days that immediately followed a night shift (Fig. 1). The subject was allowed to move away from the device briefly, but the total duration of the exposure was a full 30 min. A portable panel Lumie Brazil Seasonal Affective Disorder (SAD) Light (dimensions 50 × 31 × 15 cm, weight 2.85 kg; Lumie, Cambridge, UK) was used as a light source. This SAD Light produced white light with a warm tone at the aforementioned intensity using three 36 W broad spectrum light bulbs. It was not necessary to look at the screen for the entire 30 min, although the participants were instructed to allow light to enter freely into both eyes. At the end of the trial, a questionnaire was provided to each participant in the light therapy group to calculate the extent of overall compliance.
Statistical analysis
Clinical characteristics were reported as means and standard deviations (SDs) or frequencies and percentages for continuous and categorical variables, respectively. Normal distributions of all continuous variables were confirmed by the Kolmogorov–Smirnov test. Baseline characteristics were compared between groups using the Mann–Whitney U test or Fisher’s exact test. Intergroup comparisons of continuous variables measured at different visits were compared by an analysis of covariance (ANCOVA) with baseline values (at V0) used as a covariate. Within-group comparisons of values identified at V1 versus V0 and V2 versus V1 were performed using general linear models for repeated measures. p values < 0.05 were considered statistically significant. All analyses were performed using SPSS software program version 19.0 for Windows.
Results
The flowchart for the clinical protocol is shown in Fig. 1. As shown, we used a light treatment protocol used to treat seasonal affective disorders to determine its impact on glucose metabolism and circadian markers in a cohort of rotating NSWs.
During study protocol, no participants changed ordinary diet and physical activity. This point is crucial since intensive workout or diet changing may lead to an improving of reported quality of sleep [15]. Participants who reported only limited compliance (i.e., < 50%; n = 1) with the light therapy protocol were excluded from the data analysis. We also excluded five subjects in the control group who did not begin the study protocol after providing informed consent. Thus, 13 participants assigned to the light therapy group and nine control participants completed the study protocol and were included in the final data analysis. Three physicians (A.L., S.R., and M.B.) independently recruited and supervised the test subjects.
Notably, no participants reported any of the side effects of light therapy previously reported in the literature [16], including headache, eyestrain, nausea, insomnia, and hyperactivity. None of the outcome measures changed in the control group (Table 1). Similarly, none of the metabolic parameters, including lipids, inflammation, glucose tolerance, static (HOMA IR), and dynamic (AUC) glucose and insulin levels or blood pressure underwent significant change in those undergoing light therapy at any of the time points during the study period. Of note, we observed no significant modification of the ratio of bad/good sleepers at any time point during the protocol. Moreover, all of the rotating NSWs had fasting glucose and Hb1Ac levels within normal limits at all times during this study. However, the quantitative evaluation of gene expression in PBMCs from participants treated with light therapy revealed significantly diminished levels of REV-ERBα mRNA compared to baseline (V0 at 2.48 ± 1.54 versus V1 at 2.07 ± 0.70, p = 0.048). An additional decrease in REV-ERBα mRNA was detected after the 12-week off period (V1, 2.07 ± 0.70 versus V2, 1.47 ± 0.96, p = 0.074; Table 1 and Fig. 2). By contrast, expression levels of BMAL1 mRNA were not affected by light treatment. The REV-ERBα/BMAL1 ratio increased significantly at the first time point immediately after completion of the light therapy (V0, 1.84 ± 0.61 versus V1, 2.22 ± 0.55, p = 0.040) but dropped significantly after the wash-out period (V1, 2.22 ± 0.55 versus V2, 1.67 ± 0.57, p = 0.019; Table 1 and Fig. 2).
Discussion
Exposure to direct sunlight enables organisms to synchronize their biological systems to the external world. Circadian signaling pathways controlled by a series of transcriptional/translational feedback cycles have been identified in virtually all eukaryotic cells. The circadian transcriptional activators, BMAL1 and CLOCK, regulate the expression of thousands of transcripts. These genes also control the expression of their own repressors, REV-ERBα and REV-ERBβ, thereby creating a form of negative feedback control on circadian gene expression. As a consequence of this mechanism, the internal clock of NSWs tends to be desynchronized as they are exposed to only a limited amount of light of the appropriate solar spectrum [3]. To address this deficit, we exposed a group of active rotating NSWs to a source of warm white light for a short period each day (i.e., 30 min each morning for 12 consecutive weeks). Bright light therapy is currently in wide use for the treatment of depression as well as sleep and other neurological disorders [17, 18]. However, in our study, we did find no significant changes in glucose metabolism, insulin resistance, lipid levels, or inflammation. The lack of any clinical effect of light therapy is somehow unexpected. In fact, in a recent publication derived from the EuRhythDia project, we reported that a group of NSWs undergoing 12 weeks of light therapy significantly showed an improvement of diurnal blood pressure control, a decrease in serum glucose during OGTT and a reduction in plasma metanephrine and nor-metanephrine levels [19]. However, the participants of two studies were markedly different. In fact, respect to the above-mentioned study, the number of participants undergoing light therapy in our study was lower (n = 24 versus n = 13), whereas the mean age and the proportion of female were higher (36 ± 13 vs 39.5 ± 7.3 and 29% versusvs 54%, respectively).
On contrast, our findings revealed significant modulation of both REV-ERBα expression and the REV-ERBα/BMAL1 mRNA ratio in PBMCs isolated from otherwise healthy rotating NSWs in our experimental group. Interestingly, the role of REV-ERBα in inflammation process has been reported both in human rodent experimental models [20]. In fact, REV-ERBα regulates the timing of NLRP3 expression and production of inflammatory cytokines by macrophages, reducing the severity of peritoneal inflammation and fulminant hepatitis.
Thus, although individual gene expression levels may differ between individuals, the REV-ERBs/BMAL1 ratio might be explored further as an indicator of desynchronization and, more importantly, resynchronization of the master circadian clock due to sleep disturbances and aberrant and prolonged exposure to artificial light [21].
Notably, several recent findings highlight the roles of BMAL1 and REV-ERBα as regulators of disease severity in patients diagnosed with acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) infection [22]. In these cases, altered expression of circadian clock genes results in increased disease severity largely via increases in the extent of pathological pulmonary inflammation [23]. This is of particular importance to the health care workers, including those featured in our study. Although health care workers represent only 3% of the population, they represented 14% of all cases of Coronavirus disease-2019 (COVID-19) [24].
The role of bright light therapy in restoring appropriate circadian rhythms has been reported in studies of patients diagnosed with both Alzheimer’s and Parkinson’s disease [25]. While the mechanisms remain unclear, bright light therapy may provide relief by targeting absolute and relative expression levels of circadian clock genes. Results from previous studies performed in both humans and rodent experimental models, suggested that nocturnal light exposure has a profound impact on pathways linked to glucose metabolism, including melatonin biosynthesis [26, 27] and corticosteroid rhythmicity [28, 29]. However, the short treatment period featured in our study might conceivably result in altered REV-ERBα/BMAL1 mRNA ratios despite no discernible impact on glucose metabolism or quality of sleep.
Our study has several clear limitations. This work is a pilot study with only a few participants and a limited time frame. Thus, it may be underpowered for clinical endpoints, including modulation of glucose metabolism. Prolonged exposure to bright light may ultimately have a more substantial influence on gene expression, sleep, inflammation, and metabolism. Nonetheless, to the best of our knowledge, this is the first clinical study to highlight an important biological parameter as the direct or indirect target of an effective non-pharmacological strategy designed to address these conditions.
In conclusion, the results of our work reveal a measurable and significant effect of warm white light on the REV-ERBα/BMAL1 mRNA ratio in PMBCs of rotating NSWs. Collectively, our findings suggest that this straightforward and non-pharmacological strategy might be used to restore disruptions to the circadian clock. Future studies might address the effects of bright light in a larger population of NSWs using longer exposure periods or crossover study design to assess critical clinical variables including glucose dysregulation, sleep, and overall quality of life.
References
Parent-Thirion A, Biletta I, Cabrita J, et al. Eurofound, sixth European working conditions survey—overview report (2017 update)., Publications Office of the European Union, Luxembourg
Bureau of Labor Statistics. Workers on flexible and shift schedules in May 2004. Washington, DC 2005: US Department of Labor
Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B (2013) Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 79(6):807–811
Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES (2015) Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 38(9):1707–1713
Zoto E, Cenko F, Doci P, Rizza S (2019) Effect of night shift work on risk of diabetes in healthy nurses in Albania. Acta Diabetol 56(7):811–813. https://doi.org/10.1007/s00592-019-01307-8
Vetter C (2020) Circadian disruption: What do we actually mean? Eur J Neurosci 51(1):531–550. https://doi.org/10.1111/ejn.14255
Rizza S, Longo S, Piciucchi G et al (2020) Carotid intimal medial thickness in rotating night shift is related to IL1β/IL6 axis. Nutr Metab Cardiovasc Dis 30(10):1826–1832. https://doi.org/10.1016/j.numecd.2020.05.028 (Epub 2020 Jun 7. PMID: 32665209)
Kervezee L, Cermakian N, Boivin DB. Individual metabolomic signatures of circadian misalignment during simulated night shifts in humans. PLoS Biol 2019;18;17(6):e3000303. doi: https://doi.org/10.1371/journal.pbio.3000303. eCollection 2019 Jun.
Li Y, Gao X, Winkelman JW et al (2016) Association between sleeping difficulty and type 2 diabetes in women. Diabetologia 59(4):719–727
Rizza S, Luzi A, Mavilio M, Ballanti M, Massimi A, Porzio O, Magrini A, Hannemann J, Menghini R, Lehrke M, Staels B, Grant PJ, Boger RH, Marx N, Federici M (2021) Alterations in Rev-ERBα/BMAL1 ratio and glycated hemoglobin in rotating shift workers: the EuRhythDia study. Acta Diabetol. https://doi.org/10.1007/s00592-021-01676-z. Epub ahead of print. PMID: 33788000
Opperhuizen AL, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A (2017) Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 60(7):1333–1343
Karamitri A, Jockers R (2019) Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol 15(2):105–125. https://doi.org/10.1038/s41574-018-0130-1
Touitou Y, Reinberg A, Touitou D (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci 15(173):94–106
Cardellini M, Menghini R, Luzi A et al (2011) Decreased IRS2 and TIMP3 expression in monocytes from offspring of type 2 diabetic patients is correlated with insulin resistance and increased intima-media thickness. Diabetes 60(12):3265–3270
Bello B, Mohammed J, Useh U (2022) Effectiveness of physical activity programs in enhancing sleep outcomes among adolescents: a systematic review. Sleep Breath. https://doi.org/10.1007/s11325-022-02675-2. Epub ahead of print. PMID: 35771387
Botanov Y, Ilardi SS (2013) The acute side effects of bright light therapy: a placebo-controlled investigation. PLoS ONE 8(9):e75893. https://doi.org/10.1371/journal.pone.0075893 (PMID: 24086658; PMCID: PMC3782468)
Hirakawa H, Terao T, Muronaga M, Ishii N (2020) Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav 10(12):e01876. https://doi.org/10.1002/brb3.1876 (Epub 2020 Oct 9. PMID: 33034127; PMCID: PMC7749573)
Fishbein AB, Knutson KL, Zee PC (2021) Circadian disruption and human health. J Clin Invest 131(19):e148286. https://doi.org/10.1172/JCI148286 (PMID: 34596053; PMCID: PMC8483747)
Hannemann J, Laing A, Middleton B, Cridland J, Staels B, Marx N, Grant PJ, Federici M, Stenberg T, Skene DJ, Böger R (2021) Light therapy improves diurnal blood pressure control in night shift workers via reduction of catecholamines: the EuRhythDia study. J Hypertens 39(8):1678–1688. https://doi.org/10.1097/HJH.0000000000002848 (PMID: 33710166)
Pourcet B, Zecchin M, Ferri L et al (2018) Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice. Gastroenterology 154(5):1449–1464. https://doi.org/10.1053/j.gastro.2017.12.019 (Epub 2017 Dec 24. PMID: 29277561; PMCID: PMC5892845)
Sato S, Sakurai T, Ogasawara J et al (2014) A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol
Early JO, Menon D, Wyse CA, CervantesSilva MP, Zaslona Z, Carroll RG, PalssonMcDermott EM, Angiari S, Ryan DG, Corcoran SE et al (2018) Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci USA 115:E8460–E8468. https://doi.org/10.1073/pnas.1800431115
Ince LM, Zhang Z, Beesley S, Vonslow RM, Saer BR, Matthews LC et al (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J Off Publ Fed Am Soc Exp Biol 33:126–139. https://doi.org/10.1096/fj.201800026RR
Coppeta L, Ferrari C, Mazza A, Trabucco Aurilio M, Rizza S (2021) Factors associated with pre-vaccination SARS-CoV-2 infection risk among hospital nurses facing COVID-19 outbreak. Int J Environ Res Public Health 18(24):13053. https://doi.org/10.3390/ijerph182413053 (PMID: 34948662; PMCID: PMC8701284)
Endo T, Matsumura R, Tokuda IT, Yoshikawa T, Shigeyoshi Y, Node K, Sakoda S, Akashi M (2020) Bright light improves sleep in patients with Parkinson’s disease: possible role of circadian restoration. Sci Rep 10(1):7982. https://doi.org/10.1038/s41598-020-64645-6 (May 14PMID: 32409683; PMCID: PMC7224174)
Dauchy RT, Dauchy EM, Tirrell RP et al (2010) Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comput Med 60:348–356
Cho CH, Yoon HK, Kang SG, Kim L, Il Lee E, Lee HJ (2018) Impact of exposure to dim light at night on sleep in female and comparison with male subjects. Psych Investig 15:520–530
Dauchy RT, Wren MA, Dauchy EM et al (2015) The influence of red light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci 54:40–50
Scheer FA, Buijs RM (1999) Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab 84:3395–3398
Acknowledgements
Funding for this study was provided by the European Union’s Seventh Framework Programme for research, technological development, and demonstration under Grant Agreement No. 278397 (EuRhythDia; Targeting chronotherapeutic lifestyle intervention for diabetes and obesity to reset the circadian rhythm and improve cardiometabolic risk in the European working population) to R.H.B., N.M., B.S., M.F., and P.J.G. This work was also funded by the Ministry of University (MIUR) Progetti di Ricerca di Interesse Nazionale (PRIN) Protocol Numbers 2015MPESJS_004 and 2017FM74HK. M.L. and N.M. are funded by the Deutsche Forschungsgemeinschaft (DFG, TTR 219, M-03, M-05).
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
S.R., A.L., M.B., and A.M. performed the clinical protocols; M.M., A.M., R.M., and O.P. performed clinical chemistry and mRNA analyses; J.H., B.S., P.J.G., R.H.B., M.F., and N.M. designed the study and provided funding; S.R. performed the statistical analysis and drafted the manuscript; A.M., B.S., P.J.G., R.H.B., M.F., and N.M. revised the final full text. M.F. is the guarantor of this work.
Corresponding author
Ethics declarations
Conflict of interest
J.C. is employed by Lumie; all the other authors have not relevant conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Comitato Etico Policlinico Tor Vergata, decision #EU-FP3:125/13.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizza, S., Luzi, A., Mavilio, M. et al. Impact of light therapy on rotating night shift workers: the EuRhythDia study. Acta Diabetol 59, 1589–1596 (2022). https://doi.org/10.1007/s00592-022-01956-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01956-2