Abstract
Aims
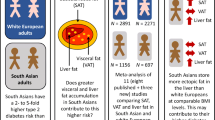
In populations of black African ancestry (BA), a paradox exists whereby lower visceral adipose tissue is found despite their high risk for type 2 diabetes (T2D). This systematic review investigates ethnic differences in other ectopic fat depots (intrahepatic lipid: IHL; intramyocellular lipid: IMCL and intrapancreatic lipid; IPL) to help contextualise their potential contribution to T2D risk.
Methods
A systematic literature search was performed in December 2020 to identify studies reporting at least one ectopic fat comparison between BA and one/more other ethnicity. For IHL, a meta-analysis was carried out with studies considered comparable based on the method of measurement.
Results
Twenty-eight studies were included (IHL: n = 20; IMCL: n = 8; IPL: n = 4). Meta-analysis of 11 studies investigating IHL revealed that it was lower in BA populations vs pooled ethnic comparators (MD −1.35%, 95% CI −1.55 to −1.16, I2 = 85%, P < 0.00001), white European ancestry (MD −0.94%, 95% CI −1.17 to -0.70, I2 = 79%, P < 0.00001), Hispanic ancestry (MD −2.06%, 95% CI −2.49 to −1.63, I2 = 81%, P < 0.00001) and South Asian ancestry comparators (MD −1.92%, 95% CI −3.26 to −0.57, I2 = 78%, P = 0.005). However, heterogeneity was high in all analyses. Most studies found no significant differences in IMCL between BA and WE. Few studies investigated IPL, however, indicated that IPL is lower in BA compared to WE and HIS.
Conclusion
The discordance between ectopic fat and greater risk for T2D in BA populations raises questions around its contribution to T2D pathophysiology in BA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the UK, type 2 diabetes (T2D) is 2–5 times more prevalent in ethnic minorities than in the general population [1], with diagnosis typically occurring 10–12 years earlier [2], and at a lower body mass index (BMI) [3]. Despite their greater risk, the pathophysiology of T2D in populations of black African ancestry (BA) remains poorly understood. Whilst it is likely that lifestyle, socioeconomic and healthcare factors play an important role [4], T2D prevalence remains higher after controlling for these factors [5]. This suggests an additional, yet unexplored, biological basis for the increased T2D risk in this population.
Current theories of T2D pathogenesis postulate that ectopic fat deposition and subsequent lipotoxicity play a central role [6, 7]. Early alterations in body fat distribution, driven by calorie excess and limited storage capacity of subcutaneous adipose tissue (SAT), result in a “spillover” of lipid to visceral adipose tissue (VAT) [8]. Not only is VAT less sensitive to the anti-lipolytic effects of insulin, resulting in greater concentrations of circulating nonesterified fatty acid (NEFA), VAT also drains directly to the liver via the portal circulation, contributing to greater intrahepatic lipid (IHL) deposition, and via lipotoxicity to hepatic insulin resistance [9, 10]. As well as increased endogenous glucose production, hepatic insulin resistance results in greater secretion of very low-density lipoprotein (VLDL) [11], in turn driving intramyocellular lipid (IMCL) and intrapancreatic lipid (IPL) accumulation, peripheral insulin resistance and impaired beta cell function [6].
Whilst these pathways appear to link calorie excess and obesity to the development of T2D in populations of white European ancestry (WE), emerging evidence suggests distinct pathways underlies the development of T2D in other ethnic groups. Interestingly, in populations of South Asian ancestry (SA), their greater susceptibility to T2D may be explained by the lower capacity of SAT to store excess lipid, leading to increased VAT and IHL in BMI-matched individuals [12]. However the same cannot be said for BA populations, as early research has found paradoxically lower VAT compared to WE [13, 14]. Considering the correlation between VAT and IHL [15], it may be hypothesised that BA would present with lower IHL than WE; and a large meta-analysis finding lower prevalence of nonalcoholic fatty liver disease (NAFLD) in BA populations supports this [16]. However, a correlation between VAT and ectopic fat depots has not always been found in BA [17]. Understanding ethnic differences in IHL, IMCL and IPL is critical in the evolution of our understanding of T2D pathophysiology in BA populations.
Therefore, our aim was to systematically review the evidence for ethnic differences in IHL, IMCL and IPL, with a focus on populations of black African ancestry.
Methods
This systematic review and meta-analysis was reported in line with the Preferred Reporting Items for Systematic Reviewers and Meta-Analysers (PRISMA) [18] and prospectively registered with PROSPERO (see: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021236093).
Information sources and search strategy
A database search was performed in Medline (Ovid), Embase (Ovid), Scopus and Cochrane CENTRAL; OpenGrey was additionally searched to identify any grey literature. An example of the full search strategy can be found in online Supplementary Table S1. Databases were searched from 1980 to 1 December 2020 (date of the search). The dates were chosen to encompass the advent of cross-sectional imaging technologies for the measurement of tissue/organ lipid deposition. No language limits were applied; however, searches were limited to human adults only (over 18 years). Reference lists of included studies were checked for further eligible studies. The full search strategy was designed to include literature investigating ethnic differences in VAT; however, this will be the subject of a separate report.
Inclusion/exclusion criteria and study selection
A modified PICO framework (Population, Intervention, Comparison, Outcome), whereby “Intervention” and “Outcome” were replaced with “phenomenon of interest”, was utilised to develop the inclusion and exclusion criteria:
Inclusion criteria: studied a distinct BA population, male or female, with any glycaemic status; included a measurement of at least one ectopic fat depot (IHL, IMCL or IPL), by computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS) or biopsy techniques (excluding IPL) and included a comparator ethnicity which included any non-BA population, male or female, with any glycaemic status. In this systematic review, participants are grouped according to their ancestry, rather the country in which they reside. For example, participants of black African ancestry include African American, Continental African and European African. Participants of South Asian ancestry (SA) included those from India, Pakistan, Bangladesh, Sri Lanka and Nepal; those of East Asian ancestry (EA) included China, Japan and Korea; those of South-east Asian ancestry included Vietnam and Malaysia.
Exclusion criteria: data for BA participants or the comparator ethnic groups were not presented separately to other ethnicities; participants were children/adolescents (< 18 years), or had any serious medical conditions/medical conditions affecting body composition or the tissue of interest; did not assess the ectopic depot using a continuous, quantitative scale, e.g. IHL comparison expressed as NAFLD prevalence (binary measure); and did not distinguish between intra- and extra-myocellular lipid.
Randomised control trials (RCTs), cohort studies and cross-sectional studies were considered for inclusion. For RCTs and cohort studies only the baseline data were included. Conference abstracts were included providing they were not duplicated in a full publication. Where cohorts were presented in more than one publication, only the publication with the largest sample size was included.
Following the removal of duplicated search results, screening was performed by one author (RR). Titles and abstracts were screened to remove studies which did not meet the inclusion/exclusion criteria. Full texts were then screened, and only studies which met the inclusion/exclusion criteria were included. Where eligibility of search results was unclear, a decision was reached via discussions between authors (RR, LG and MW).
Data extraction and quality assessment
One author (RR) extracted data from the selected studies using Microsoft Excel. Data extracted included publication author and year, name of the cohort, number and ethnicity of BA participants, ethnicity and number of comparator participants, sex, age, BMI, glycaemic status, ectopic fat comparison, methodology of ectopic fat measurement and statistical information. Unadjusted means and standard deviation (SD) were extracted in the first instance or covariate adjusted means and SD where unadjusted were not reported. Where data were missing, at least two attempts were made to contact the corresponding authors. Where authors did not respond, the publication was included in the narrative analysis only.
The Newcastle–Ottawa Quality assessment Scale (NOS) for cohort studies, adapted to assess cross-sectional studies, was used to assess study quality [19]. This was modified where domains were not appropriate, either amending or removing the domain; hence, the maximum score was 8*. See online Supplementary Table S2 for the modified scale. Briefly, the “nonrespondents” domain was removed, and “ascertainment of the exposure” was modified to increase its applicability to ethnicity.
Meta-analyses
Although meta-analysis was not planned for any outcome (see: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021236093), following the data extraction it was apparent that meta-analysis for IHL was appropriate. Studies which measured IHL by magnetic resonance methodologies (MRI and MRS), and were comparable according to how IHL was calculated and presented, were included in meta-analyses. Meta-analysis was not conducted for studies investigating IMCL due to half of the data being presented as median and interquartile range (IQR), and for IPL due to the small number of studies (n = 4). Therefore, the results for IMCL and IPL are presented in a narrative summary.
Means and SD were used in the meta-analysis; hence, data presented as standard error or 95% confidence intervals (CI) were converted to SD and mean and SD were approximated from medians and IQR (converted in 4 studies) [20, 21]. Furthermore, in mixed-sex studies where IHL data were presented according to sex, samples were combined to give a single group per study [20]. Where a single publication had more than one ethnic comparator, the n was divided by the number of ethnic comparators and rounded down to the nearest whole number [22]. The publication by Alenaini et al. [23] included data from two distinct cohorts, which were included in the meta-analysis separately.
Fixed effects models were used to calculate estimates of pooled mean differences and 95% CI for IHL between BA and comparator ethnicity participants, and heterogeneity was assessed using the I2 statistic. Heterogeneity within the BA versus WE comparison was investigated by subgroup analysis of age (30–39 years, 40–49 years, 50–59 years, 60+ years), sex (male, female, mixed-sex), BMI (25.0–29.9 kg/m2, 30.0–34.9 kg/m2, 35.0–40.0 kg/m2) and glycaemic status (nondiabetic, T2D, mixed glycaemic status, not reported). Subgroup analyses were not performed for other comparisons (i.e. BA vs. other comparator ethnicity groups), due to the small numbers of studies included in these comparisons. All data analyses and figure production were performed in Review Manager 5.4 (The Cochrane Collaboration, 2020).
Sensitivity analyses were performed to assess the effect of including the following data in the meta-analysis: estimated means and SD from median and IQR, and covariate adjusted means and SD. Finally, publication bias was assessed by inspection of funnel plots for asymmetry.
Results
A total of 2047 studies were identified from the search. Following screening, 28 studies were considered eligible (Fig. 1). The majority of studies were conducted in African American populations (n = 17), followed by black European (n = 7), black South African (n = 2), African American and African Immigrant (n = 1) and BA participants sampled worldwide (n = 1). Sample sizes of the of BA participants ranged between n = 15 [24] and n = 1893 [25]. See Table 1.

Adapted from PRISMA [18]. NAFLD; nonalcoholic fatty liver disease. Visceral adipose was excluded at this step, due to the volume of studies for inclusion in future report (*)
Flow chart of study screening and selection.
Of the eligible studies, 15 included mixed-gender cohorts, six were male only and seven were female only. Most studies (n = 11) were conducted in participants without T2D, one was in participants with prediabetes, three in participants with T2D, and the remaining either not reported or a range of glycaemic status. The mean BMI of the whole cohorts equated to normal weight (20.0–24.9 kg/m2) in one study, overweight (25.0–29.9 kg/m2) in 12 studies and obese (> 30.0 kg/m2) in 15 studies (Table 1).
Scores for quality assessment ranged from 2* to 8*, with all but two studies scoring more than 50% (4*). Studies typically lost stars for the “Representativeness of the sample” (n = 11), “Sample size justification” (n = 18) and “Assessment of the outcome” (n = 24) domains (online Supplementary Table S2).
Intrahepatic lipid
Twenty studies assessed IHL; using: MRS (n = 8) [23, 24, 26,27,28,29,30,31], CT (n = 7) [25, 32,33,34,35,36,37], MRI (n = 4) [38,39,40,41] and both MRS and CT (n = 1) [42]. In the majority of these studies, the comparator ethnicities were WE (n = 18) [23,24,25,26,27,28,29,30,31,32,33,34,35, 37,38,39,40, 42] and HIS (n = 7) [25, 28, 31, 34, 36, 40, 41], with smaller numbers of SA (n = 3) [23, 25, 33], EA (n = 3) [25, 34, 40], South-east Asian (n = 1) [34] and Native Hawaiian (n = 1) [40].
Of the 18 studies comparing IHL between BA and WE, 11 studies found statistically significantly lower IHL [25, 27, 29, 30, 32,33,34, 37,38,39, 42], and two further studies found a nonstatistically significant trend towards lower IHL [24, 26] in BA. Two further studies found that differences in IHL were dependent on sex, with BA exhibiting lower IHL compared to WE in males only [28] and females only [40], where a sex-by-ethnicity comparison was performed. A further study assessing ethnic differences in IHL across two different cohorts found that IHL was lower in BA women only within the Hammersmith cohort, with no statistically significant differences in the UK Biobank cohort [23].
All studies comparing IHL between BA and HIS found statistically significantly lower IHL in BA (n = 7) [25, 28, 31, 34, 36, 40, 41].
Of the three studies comparing IHL between BA and SA, two found statistically significantly lower IHL in BA [25, 33]. The third study assessing ethnic differences in IHL across two different cohorts found that IHL was lower in BA in women only within the Hammersmith cohort, with no statistically significant differences in the UK Biobank cohort [23].
Of the three studies comparing IHL between BA and EA, one study found statistically significantly lower IHL in BA [40]. The remaining two studies found no statistically significant differences (n = 2) [25, 34].
Data from 11 studies that used MRI or MRS [23, 24, 27,28,29,30,31, 38,39,40,41] were included in a meta-analysis (2 studies could not be included due to the method of IHL calculation or presentation [26, 42]). The pooled effect of BA ethnicity on IHL is presented in Fig. 2. Compared to other ethnicities as a pooled comparator, BA presented with significantly lower IHL (MD −1.35%, 95% CI −1.55 to −1.16, I2 = 85%, P < 0.00001). In subgroup analyses, IHL was significantly lower in BA compared to WE (MD −0.94%, 95% CI −1.17 to -0.70, I2 = 79%, P < 0.00001), HIS (MD −2.06%, 95% CI −2.49 to −1.63, I2 = 81%, P < 0.00001) and SA (MD −1.92%, 95% CI −3.26 to −0.57, I2 = 78%, P = 0.005). Furthermore, test for subgroup differences between comparator ethnicities was statistically significant (P = 0.00001). Overall, heterogeneity was high (I2 > 75%); this heterogeneity could not be explained by subgroup analyses for sex, age, BMI category or glycaemic status when comparing BA and WE (see online Supplementary Fig. S3-6). This suggests that IHL seems to be significantly lower in BA compared to comparator ethnicities regardless of these factors, but due to this unexplained heterogeneity, the magnitude of the difference in IHL between BA and comparator ethnicities is uncertain.
Forest plot for the effect of Black African ancestry (BA) on intrahepatic lipid (IHL). Studies utilised magnetic resonance techniques, when compared to white European ancestry, Hispanic ancestry, South Asian ancestry and East Asian ancestry. Data are presented as mean difference (% IHL) with 95% confidence intervals (CI) for individual studies and pooled estimates. SD: standard deviation
In sensitivity analyses, removing studies where means and SD were estimated [24, 28, 29, 38] and those only reporting means adjusted for covariates [23, 40] changed the magnitude but not the direction of the difference in IHL between BA and comparator ethnicities: see online Supplementary Fig. 7–8.
Manual inspection of the funnel plot (online Supplementary Fig. S9) revealed observable asymmetry in the right of the plot which could be suggestive of publication bias.
Intramyocellular lipid
Eight studies investigated ethnic differences in IMCL (summarised in Table 2), all focused on WE as the comparator ethnicity (n = 8). In these studies, IMCL was mostly assessed using MRS in 6 studies [24, 29, 30, 43,44,45] and with biopsy methods in 2 studies [46, 47].
Five studies found no significant ethnic differences [30, 43,44,45,46]. Two studies found significant IMCL ethnic differences with one reporting higher IMCL in BA compared to WE [29], whereas the other found BA to have significantly lower IMCL in type I fibres and a trend for lower IMCL in type II fibres [47]. A further study found a trend for BA having lower IMCL compared to WE in the tibialis anterior, but not the soleus [24].
Intrapancreatic lipid
Ethnic differences in IPL were the least investigated (n = 4), with three studies using MRI [17, 48, 49] and one using MRS [31] (Table 2). Comparator ethnicities included WE in 3 studies [17, 31, 48] and HIS in 2 studies [31, 49]. In the three studies investigating differences in IPL between BA and WE, two found lower IPL in BA [31, 48] and one study found no significant differences [17]. In the two studies investigating differences in IPL between BA and HIS, both studies found lower IPL in BA [31, 49].
Discussion
This systematic review assessed ethnic differences in ectopic fat with a focus on BA populations. Our meta-analysis provides evidence that BA populations have lower IHL compared to other ethnic groups, in both sexes, across all ages and BMI categories and independent of glycaemic status. For IMCL, most studies found no differences between BA and WE, which was the only ethnic comparator. For IPL, there were a notable lack of studies; however, those included suggest there may be lower IPL in BA compared to both WE and HIS populations.
Deposition of ectopic fat, particularly in the liver, skeletal muscle and pancreas, has been proposed to be instrumental in the development of T2D, driving insulin resistance and beta cell dysfunction [6]. Whilst VAT is proposed to be an important correlate of ectopic fat accumulation as a result of NEFA “spillover” [9], a review of ethnic differences in VAT was beyond the scope of this review. However, it is well recognised in the literature that BA have a more favourable body fat distribution, with less VAT despite similar SAT compared to other ethnicities [17, 34, 50]. Considering the close relationship between VAT and IHL [15], lower IHL in BA populations would also therefore be expected. Most studies in our review found lower IHL in BA compared to other ethnicities, particularly WE, HIS and SA. Our finding is supported by previous meta-analyses, which have found lower NAFLD prevalence in BA compared to WE, HIS and Asian populations [16, 51]. IHL is proposed to contribute to the development of T2D [52], through its effect on hepatic insulin sensitivity [9], and has been shown to be a significant predictor of T2D in both WE and SA populations [53, 54]. There is a paradox in BA populations who are recognised to have a disproportionately high risk of T2D [1], despite our finding of significantly lower IHL (and arguably lower IPL) levels. Consistent with lower IHL in BA, it would be plausible to expect greater hepatic insulin sensitivity. Whilst several studies have investigated ethnic differences in whole-body insulin sensitivity [24, 35, 43], these have mostly used methodologies that do not differentiate between “whole-body” and tissue-specific/multiorgan (liver, skeletal muscle, adipose tissue) insulin sensitivity. In the few studies that have attempted to differentiate organ-specific measurements, surprisingly, hepatic insulin sensitivity has been found to be similar between BA and WE women [54, 55], although this is not a consistent finding [24, 56]. Less work has been conducted in men, but recent studies have found similar hepatic insulin sensitivity between BA and WE with and without T2D [43, 57]. Whilst more research is needed to clarify these inconsistent findings, current evidence suggests that despite lower IHL, BA populations have paradoxically similar hepatic insulin sensitivity. To determine the importance of IHL on hepatic insulin sensitivity in BA populations, ethnic differences in this relationship have been investigated. In WE, a significant negative association has been consistently reported [9, 38, 43], however, the relationship is less clear amongst BA. In women, a significant negative association is reported [24, 55], but in men no associations have been found [38, 39]. Whilst these contradictory findings may be explained by differences in the BMI or glycaemic status of the participants, another possibility is that there is sexual dimorphism in the pathophysiology of T2D within BA populations. If this is the case, these findings may suggest that BA women are more sensitive to the lipotoxic effects of IHL, such that lower IHL can initiate the same deleterious effects. In contrast, there may be an uncoupling of IHL and hepatic insulin sensitivity in BA men, suggesting other mechanisms are more important determinants of hepatic insulin sensitivity.
If existing theories of ectopic fat deposition are applicable to BA populations, we would also expect a reduction in IMCL and IPL deposition [9, 11]. We found fewer studies investigating IMCL, and they were limited to a single comparative ethnicity (WE). Nevertheless, our findings suggest IMCL does not differ between BA and WE, which is supported by work in adolescents [58, 59]. Two studies found significant ethnic differences in IMCL [29, 47], but these may be explained by ethnic differences in habitual physical activity or muscle fibre type. Ethnic differences in muscle fibre type are rarely taken into consideration when investigating differences in IMCL. However, BA are reported to have less type 1 and more type 2 fibres [60], and the extent of lipid deposition appears to be related to fibre type [61]. Therefore, until studies control for fibre type, observations of IMCL differences between ethnicities cannot be interpreted with any certainty. The role of IMCL in peripheral insulin sensitivity is controversial, regardless of ethnicity. Lipid metabolites (diacylglycerol, ceramides) are now proposed to be more important drivers of peripheral insulin resistance [62]. Regardless of sex, studies have failed to find an association between IMCL and peripheral insulin sensitivity in BA [43, 45, 47], suggesting an uncoupling of peripheral insulin sensitivity to IMCL. However, further research is required to determine the importance of IMCL, and perhaps lipid metabolites, in peripheral insulin sensitivity and T2D risk in BA populations.
Very few studies examined IPL; in the four studies identified, three found lower IPL in BA compared to other ethnicities (WE and HIS) [31, 48, 49]. The fourth study found no significant ethnic differences, although this may be explained by the lower BMI of the participants [17]. IPL is negatively associated with markers of beta cell function in WE [17, 63]. However, it is unclear if the same is true for BA populations, as reported associations between IPL and beta cell function are inconsistent [17, 31]. Differences in findings may be attributed to the differences in methodologies used to assess beta cell function. Interestingly, following the onset of T2D, IPL is not associated with beta function in WE or BA [48, 64], suggesting that factors other than IPL are responsible for the progressive dysfunction.
Ethnic differences in the relationships between ectopic fat depots are relatively under-reported. Leading theories of ectopic fat deposition and T2D suggest interrelationships between ectopic fat depots [8], which is supported by studies in WE populations [17]. However, when ectopic fat depots have been measured in BA populations, differences in the associations between them have been found. To summarise, IHL is unrelated to VAT, IMCL or IPL in men [17], and only related to IPL in women [65]. Although the evidence is limited, it appears the mechanisms of ectopic fat deposition may differ in BA. Ectopic fat deposition is thought to be a downstream consequence of dysregulated lipid trafficking. Despite the more favourable fasting lipid profile which is historically reported in BA [66], this population have been found to exhibit greater postprandial lipaemia [67], which is indicative of dysregulated lipid trafficking. Lower ectopic fat deposition (IHL), despite greater postprandial lipaemia, presents a further paradox which may support distinct mechanisms of ectopic fat deposition in BA populations. Determining these mechanisms, and how they interact with cardiometabolic risk, is an interesting and important avenue for future research.
The limitations of our review warrant consideration. Generally, studies scored well on the modified NOS, suggesting that the strength of the evidence at the study level was good. However, manual inspection of the funnel plot revealed asymmetry. Heterogeneity and extreme results are more common in nonrandomised study designs, which may partly explain the distribution of results on the funnel plot. Searching for unpublished observational studies is more challenging than RCTs, as observational studies tend not to be registered as often as RCTs. Therefore, this asymmetry may represent publication bias.
In conclusion, IHL is consistently reported to be lower in BA populations compared to other ethnicities, particularly WE, HIS and SA. Our subgroup analyses found this difference to be independent of age, sex, BMI and glycaemic status but substantial heterogeneity was present within our analyses, therefore the magnitude of the difference in IHL between BA and comparator ethnicities is uncertain. IPL also appears to be lower in BA compared to WE and HIS, but more research is required before conclusions can be drawn. Despite these differences, IMCL does not appear to be different between BA and WE; however, studies controlling for muscle fibre type and investigating lipid metabolites will be of great interest. Differences in ectopic fat depots in BA, together with their differing relationship between organ lipid content and insulin sensitivity, supports a distinct T2D pathophysiology compared to other ethnic groups. Further research should aim to identify the interrelationships between these depots and how the mechanisms of lipid deposition are different in this population. In addition, identifying how ectopic fat depots are related to whole-body and tissue-specific insulin sensitivity in BA is important to explore the disproportionate risk that this population experiences.
References
Sproston K, Mindell J (2006) Health survey for England 2004. The health of minority ethnic groups
Paul SK, Owusu Adjah ES, Samanta M et al (2017) Comparison of body mass index at diagnosis of diabetes in a multi-ethnic population: a case-control study with matched non-diabetic controls. Diabetes Obes Metab 19(7):1014–1023. https://doi.org/10.1111/dom.12915
Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP (2014) Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care 37(9):2500–2507
Goff LM (2019) Ethnicity and T2D in the UK. Diabet Med 36(8):927–938
Pham TM, Carpenter JR, Morris TP, Sharma M, Petersen I (2019) Ethnic differences in the prevalence of T2D diagnoses in the UK: cross-sectional analysis of the health improvement network primary care database. Clin Epidemiol 11:1081
Cusi K (2010) The role of adipose tissue and lipotoxicity in the pathogenesis of T2D. Curr DiabRep 10(4):306–315
Weinberg J (2006) Lipotoxicity. Kidney Int 70(9):1560–1566
Lewis GF, Carpentier A, Adeli K, Giacca A (2002) Disordered fat storage and mobilization in the pathogenesis of insulin resistance and T2D. Endocr Rev 23(2):201–229
Gastaldelli A, Cusi K, Pettiti M et al (2007) Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133(2):496–506
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD (2004) Splanchnic lipolysis in human obesity. J Clin Invest 113(11):1582–1588. https://doi.org/10.1172/jci21047
Choi SH, Ginsberg HN (2011) Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22(9):353–363. https://doi.org/10.1016/j.tem.2011.04.007
Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A (2007) Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol 36(1):220–225
Carroll JF, Chiapa AL, Rodriquez M et al (2008) Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity 16(3):600–607
Perry AC, Applegate EB, Jackson ML et al (2000) Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol 89(2):636–643
Korenblat KM, Fabbrini E, Mohammed BS, Klein S (2008) Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134(5):1369–1375
Rich NE, Oji S, Mufti AR et al (2018) Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 16(2):198–210. e192
Hakim O, Bello O, Ladwa M et al (2019) Ethnic differences in hepatic, pancreatic, muscular and visceral fat deposition in healthy men of white European and black west African ethnicity. Diabetes Res Clin Pract 156:107866
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á (2013) Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 13(1):1–17
Higgins JPT LT, Deeks JJ (eds) (2021) Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):1–13
Higgins JPT ES, Li T (eds) (2021) Chapter 23: including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook
Alenaini W, Parkinson JRC, McCarthy JP et al (2020) Ethnic differences in body fat deposition and liver fat content in two UK-based cohorts. Obesity 28(11):2142–2152. https://doi.org/10.1002/oby.22948
Goedecke JH, Keswell D, Weinreich C et al (2015) Ethnic differences in hepatic and systemic insulin sensitivity and their associated determinants in obese black and white South African women. Diabetologia 58(11):2647–2652. https://doi.org/10.1007/s00125-015-3720-7
Garg SK, Lin F, Kandula N et al (2016) Ectopic fat depots and coronary artery calcium in South Asians compared with other racial/ethnic groups. J Am Heart Assoc 5(11):e004257. https://doi.org/10.1161/JAHA.116.004257
Allister-Price C, Craig CM, Spielman D, Cushman SS, McLaughlin TL (2019) Metabolic markers, regional adiposity, and adipose cell size: relationship to insulin resistance in African-American as compared with Caucasian women. Int J Obes 43(6):1164–1173. https://doi.org/10.1038/s41366-018-0191-1
Bril F, Portillo-Sanchez P, Liu IC, Kalavalapalli S, Dayton K, Cusi K (2018) Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care 41(1):187–192. https://doi.org/10.2337/dc17-1349
Browning JD, Szczepaniak LS, Dobbins R et al (2004) Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40(6):1387–1395. https://doi.org/10.1002/hep.20466
Chung ST, Cravalho CKL, Meyers AG et al (2020) Triglyceride paradox is related to lipoprotein size, visceral adiposity and stearoyl-CoA desaturase activity in black versus white women. Circ Res. https://doi.org/10.1161/CIRCRESAHA.119.315701
Marlatt KL, White UA, Beyl RA et al (2018) Role of resistant starch on diabetes risk factors in people with prediabetes: design, conduct, and baseline results of the STARCH trial. Contemp Clin Trials 65:99–108. https://doi.org/10.1016/j.cct.2017.12.005
Szczepaniak LS, Victor RG, Mathur R et al (2012) Pancreatic steatosis and its relationship to beta-cell dysfunction in humans: racial and ethnic variations. Diabetes Care 35(11):2377–2383. https://doi.org/10.2337/dc12-0701
Brown JA, Hames KC, Jakicic JM, Delany JP, Goodpaster BH (2009) Cardiometabolic risk factors in severely obese African-American and Caucasian women. Diabetes 58(SUPPL. 1A)
Naran NH, Haagensen M, Crowther NJ (2018) Steatosis in South African women: How much and why? PLoS ONE 13(1):e0191388. https://doi.org/10.1371/journal.pone.0191388
Nazare J-A, Smith JD, Borel A-L et al (2012) Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am J Clin Nutr 96(4):714–726
North KE, Graff M, Franceschini N et al (2012) Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. Eur J Gastroenterol Hepatol 24(1):9–16. https://doi.org/10.1097/MEG.0b013e32834a94fb
Wagenknecht LE, Palmer ND, Bowden DW et al (2011) Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver Int 31(3):412–416. https://doi.org/10.1111/j.1478-3231.2010.02444.x
Whitaker KM, Pereira MA, Jacobs DR, Sidney S, Odegaard AO (2017) Sedentary behavior, physical activity, and abdominal adipose tissue deposition. Med Sci Sports Exerc 49(3):450–458. https://doi.org/10.1249/MSS.0000000000001112
Hakim O, Bello O, Bonadonna RC et al (2019) Ethnic differences in intrahepatic lipid and its association with hepatic insulin sensitivity and insulin clearance between men of black and white ethnicity with early T2D. Diabetes Obes Metab 21(9):2163–2168. https://doi.org/10.1111/dom.13771
Ladwa M, Bello O, Hakim O et al (2020) Insulin clearance as the major player in the hyperinsulinaemia of black African men without diabetes. Diabetes Obes Metab 22(10):1808–1817. https://doi.org/10.1111/dom.14101
Lim U, Monroe KR, Buchthal S et al (2019) Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology 156(4):966. https://doi.org/10.1053/j.gastro.2018.11.021
Walker RW, Le K-A, Davis J et al (2012) High rates of fructose malabsorption are associated with reduced liver fat in obese African Americans. J Am Coll Nutr 31(5):369–374
Larson-Meyer DE, Newcomer BR, Heilbronn LK et al (2008) Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring, MD) 16(6):1355–1362. https://doi.org/10.1038/oby.2008.201
Bello O, Ladwa M, Hakim O et al (2020) Differences in the link between insulin sensitivity and ectopic fat in men of Black African and White European ethnicity. Eur J Endocrinol 182(1):91–101. https://doi.org/10.1530/EJE-19-0636
Hakim O, Charles-Edwards G, Whitcher B, Shuaib H, Goff LM (2017) Intramyocellular lipid and its relationship with insulin sensitivity and fat in type 2 diabetic men of White and Black ethnicity. Proc Nutr Soc 76(OCE4). https://doi.org/10.1017/S0029665117002853
Ingram KH, Lara-Castro C, Gower BA et al (2011) Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity (Silver Spring, MD) 19(7):1469–1475. https://doi.org/10.1038/oby.2011.45
DeLany JP, Dube JJ, Standley RA et al (2014) Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the absence of obesity. J Clin Endocrinol Metab 99(11):4307–4314. https://doi.org/10.1210/jc.2014-2512
Smith LM, Yao-Borengasser A, Starks T, Tripputi M, Kern PA, Rasouli N (2010) Insulin resistance in African-American and Caucasian women: differences in lipotoxicity, adipokines, and gene expression in adipose tissue and muscle. J Clin Endocrinol Metab 95(9):4441–4448. https://doi.org/10.1210/jc.2010-0017
Hakim O, Bonadonna RC, Mohandas C et al (2019) Associations between pancreatic lipids and β-cell function in Black African and White European men with T2D. J Clin Endocrinol Metab 104(4):1201–1210. https://doi.org/10.1210/jc.2018-01809
Le K-A, Ventura EE, Fisher JQ et al (2011) Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 34(2):485–490. https://doi.org/10.2337/dc10-0760
Carroll JF, Fulda KG, Chiapa AL et al (2009) Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity 17(7):1420–1427. https://doi.org/10.1038/oby.2008.657
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84
DeFronzo RA (2009) From the triumvirate to the „ominous octet”: a new paradigm for the treatment of T2D mellitus. Clin Diabetol 10(3):101–128
Narayan kv, KANDULA NR, Liu K, Kanaya AM (2019) 1636-P: incidence and predictors of diabetes and prediabetes among South Asians in the United States: the MASALA study. In Am Diabetes Assoc
Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE (2004) Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabet Med 21(10):1090–1095. https://doi.org/10.1111/j.1464-5491.2004.01290.x
Chung ST, Courville AB, Onuzuruike AU et al (2018) Gluconeogenesis and risk for fasting hyperglycemia in Black and White women. JCI Insight 3(18):e121495. https://doi.org/10.1172/jci.insight.121495
Ellis AC, Alvarez JA, Granger WM, Ovalle F, Gower BA (2012) Ethnic differences in glucose disposal, hepatic insulin sensitivity, and endogenous glucose production among African American and European American women. Metabolism 61(5):634–640
Bello O, Mohandas C, Shojee-Moradie F et al (2019) Black African men with early T2D have similar muscle, liver and adipose tissue insulin sensitivity to white European men despite lower visceral fat. Diabetologia 62(5):835–844
Lee S, Boesch C, Kuk JL, Arslanian S (2013) Effects of an overnight intravenous lipid infusion on intramyocellular lipid content and insulin sensitivity in African-American versus Caucasian adolescents. Metabolism 62(3):417–423
Liska D, Dufour S, Zern TL et al (2007) Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE 2(6):e569
Tanner CJ, Barakat HA, Dohm GL et al (2002) Muscle fiber type is associated with obesity and weight loss. Am J Physiol-Endocrinol Metab 282(6):E1191–E1196. https://doi.org/10.1152/ajpendo.00416.2001
Chow LS, Mashek DG, Wang Q, Shepherd SO, Goodpaster BH, Dubé JJ (2017) Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type-specific IMCL accumulation. J Appl Physiol 123(1):71–78. https://doi.org/10.1152/japplphysiol.00209.2017
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148(5):852–871
Heni M, Machann J, Staiger H et al (2010) Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev 26(3):200–205. https://doi.org/10.1002/dmrr.1073
Tushuizen ME, Bunck MC, Pouwels PJ et al (2007) Pancreatic fat content and β-cell function in men with and without T2D. Diabetes Care 30(11):2916–2921
Fortuin-de Smidt MC, Mendham AE, Hauksson J et al (2021) β-cell function in black South African women: exploratory associations with insulin clearance, visceral and ectopic fat. Endocr Connect 10(5):550–560
Després J-P, Couillard C, Gagnon J et al (2000) Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20(8):1932–1938
Goff LM, Whyte MB, Samuel M, Harding SV (2016) Significantly greater triglyceridemia in Black African compared to White European men following high added fructose and glucose feeding: a randomized crossover trial. Lipids Health Dis 15(1):1–9
Acknowledgements
The authors would like to thank Olah Hakim for her support during the conception and design of the study, as well as Karen Poole for her assistance in building the search strategy.
Funding
This work was funded by the King’s Medical Research Trust, Joint Research Committee (JRC) PhD Studentship.
Author information
Authors and Affiliations
Contributions
The key contributions are: RR, MW and LG conceived and designed the study; RR, MW, LG, SN, DC and GK were involved in the protocol development and approach; RR and SN collected and analysed the data; and RR drafted the manuscript. All authors read, revised and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reed, R.M., Nevitt, S.J., Kemp, G.J. et al. Ectopic fat deposition in populations of black African ancestry: A systematic review and meta-analysis. Acta Diabetol 59, 171–187 (2022). https://doi.org/10.1007/s00592-021-01797-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01797-5