Abstract
Aims
Periodontal disease (PD) is a chronic inflammation of periodontal tissue associated with infection from specific anaerobic pathogens contained in dental plaque. Both type 1 and type 2 diabetes are associated with an increased prevalence of PDs. A two-way relationship between diabetes and periodontitis has been proposed, with diabetes increasing the risk for periodontitis, and periodontal inflammation negatively affecting glycaemic control. To date, the relationship between PD and glucose variability in type 1 diabetes has not been evaluated. To investigate the prevalence of PD in patients with type 1 diabetes and its association with glycemic control and glucose variability.
Methods
In this cross-sectional study, all enrolled patients were scheduled to attend both a diabetologic and a periodontal visit. HbA1c, glucose coefficient of variation (CV), loss of clinical attachment (CAL), and periodontal probing depth (PPD) were collected.
Results
136 patients were included in the analysis. The prevalence of PD was 63%. A significant correlation was found between mean CAL and glucose CV (r = 0.31, p = 0.002), but not with HbA1c. Mean PPD was also associated with glucose CV (r = 0.27 and 0.044), but not with HbA1c. In a multiple linear regression model, with mean CAL as dependent variable, age, glucose CV, and smoking habit resulted significantly associated (r = 0.23, p = 0.013; r = 0.33, p = 0.001; r = 0.34, p < 0.001, respectively). Assuming mean PPD as dependent variable, multiple linear regression analysis showed a significant association with glucose CV and smoking habits only.
Conclusions
PD is associated with glucose variability in patients with type 1 diabetes also after adjusting for the main confounders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease (PD) is a chronic inflammation of periodontal tissue associated with infection from specific anaerobic pathogens contained in dental plaque [1]. The progressive destruction of connective tissue attachment and bone support can result in tooth loss and systemic inflammation [2]. PD affects over 30% of adults with severe forms in more than 10% of the population [3].
Both type 1 and type 2 diabetes are associated with an increased prevalence of PD [4, 5]. In addition, among patients with diabetes, a higher HbA1c level is associated with a higher prevalence and severity of PD [6]. It is conceivable that hyperglycemia, through the activation of oxidative stress pathway, induces periodontal damage by exerting detrimental effects in the periodontium as in several other well-known organs [7]. It is also possible that the systemic inflammation determined by PD alters glucose control [8]. Systemic and local changes of pro-inflammatory cytokine levels (i.e., IL-1β, receptor activator of nuclear factor-Kappa B ligand/osteoprotegerin, and IL-6) are noted in both type 1 and type 2 diabetic individuals [9].
The reduction of glucose variability is emerging as a primary goal in the treatment of type 1 diabetes [10]. The relationship between PD and glucose variability has not been extensively studied so far. Aim of the present study is the assessment of the correlation between glucose variability and periodontal disease in type 1 diabetes.
Material and Methods
The PARODIA Project is a single-centre, cross-sectional study aimed at investigating the prevalence of periodontal disease (PD) in patients with type 1 diabetes and its association with glycemic control and glucose variability.
Type 1 diabetic patients aged ≥ 18 years currently treated with multiple daily insulin injections or continuous subcutaneous insulin infusion, who provided their written informed consent, were enrolled if they had been continuously using for the last three months the FreeStyle Libre Flash Glucose Monitoring (FGM) system (Abbott Diabetes Care, IL), which is fully reimbursed for type 1 diabetes in Tuscany, and the most widely used system for interstitial glucose monitoring in those patients. Subjects with a history of cancer, HIV, bone metabolic disease, history of radiation, or immunosuppressive/modulating therapy were excluded, as well as those who had taken antibiotics, corticosteroids, or non-steroidal anti-inflammatory drugs in the previous 3 months, in order to avoid the interference of confounders.
All patients underwent a standardized measurement of HbA1c (HPLC-assay, Menarini Diagnostic, Florence, Italy). At the Adult Diabetes Clinic in Florence, screening for chronic diabetes-related complications, lipid profile assay, weight, and blood pressure measurements were performed at enrolment, unless available in the last 12 months. FGM data were downloaded using the cloud-based Libreview® system and used for the calculation of glucose coefficient of variation (CV), percentage time with glucose in target range (TIR), in hyperglycemia (> 10 mmol/L) and hypoglycemia (< 3.9 mmol/L).
Clinical evaluation of periodontal disease was done according to international standards [11]. A full-mouth periodontal examination was performed at the Research Unit in Periodontology and Periodontal Medicine, by a single operator using a NCP15 periodontal probe, collecting data (six sites/tooth of each subject) on periodontal probing depths (PPD, as the distance between the gingival margin and the apical limit of gingival crevice) and gingival recession (REC, as the distance between the cemento-enamel junction and the gingival margin). The amount of loss clinical attachment (CAL) for each assessed site was estimated as PPD + REC. Periodontitis case definition system was used to differentiate stage I to IV periodontitis [12].
The primary endpoint was the correlation of severity of PD in patients with type 1 diabetes, as assessed through CAL, with glucose variability, expressed by glucose CV. Assuming an estimated PD prevalence of 25% [13] and with a precision of 7%, the projected sample size was of 150 patients. Descriptive statistics was performed calculating mean ± Standard Deviation (SD) and median [quartiles], depending on data distribution. Associations between age, sex, HbA1c, diabetes duration, glucose CV, mean CAL, and smoking habits were explored calculating Spearman’s correlations. Linear regression models were applied for multivariate analyses.
In the multiple linear regression analysis, mean CAL or glucose CV, respectively, will be considered as the dependent variable, whereas age, glucose CV, or mean CAL and smoking habit as the independent ones. All analyses were performed using SPSS 26.
The study methodology was designed in guidance by STROBE guideline for cross-sectional study.
Results
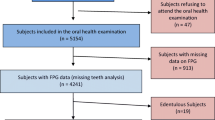
Of the 150 enrolled patients, 136 attended the scheduled periodontal visit and were therefore included in analysis. The characteristics were not significantly different from those of the enrolled population (Table 1). The prevalence of PD, according to the diagnostic criteria specified above, was 63% (stage I n = 14; stage II n = 20; stage III n = 43; Stage IV n = 9). Periodontal parameters are reported in Table 1.
A significant correlation was found between mean CAL and glucose CV (r = 0.31, p = 0.002; Fig. 1 Panel A), but not HbA1c (r = 0.038 p = 0.673). Mean PPD was also associated with glucose CV, but not with HbA1c (r = 0.27 and 0.044; p = 0.007 and 0.619, respectively; Fig. 1 Panel B). In a multiple linear regression model, with mean CAL as dependent variable, age, glucose CV, and smoking habit resulted significantly associated (r = 0.23, p = 0.013; r = 0.33, p = 0.001; r = 0.34, p < 0.001, respectively). Assuming mean PPD as dependent variable, multiple linear regression analysis showed a significant association with glucose CV and smoking habits only (r = 0.23, p = 0.019; r = 0.33, p = 0.001, respectively).
An alternative model, with glucose CV as dependent variable, showed a significant association of mean CAL with glucose CV, even after adjusting for age and smoking habits (r = 0.29, p = 0.013) and significant association of mean PPD with glucose, even after adjusting for smoking habits (r = 0.26, p = 0.029). No correlation was found with TIR, time in hypoglycemia and in hyperglycemia (data not shown).
Discussion
The prevalence of PD in this study was higher than that reported in recently published meta-analysis of cross-sectional studies [4]. This difference could be due, at least partly, to differences in assessment methods, diagnostic, and classification criteria. Notably, studies with more accurate assessment methods, similar to those used in the present survey, provided higher prevalence estimates [14, 15]. However, differences across genetically, geographically, and culturally heterogeneous populations cannot be ruled out.
A higher glucose variability, estimated as glucose CV, was associated with both mean CAL (which is an expression of PD-related bone loss) and mean PPD (which indicates local inflammation).
To our knowledge, this is the first available report of such an association. Those associations were confirmed at multivariate analyses after adjusting for the main confounders, i.e., smoking and age. Conversely, no significant association of either mean PPD or mean CAL was observed with HbA1c. Although this latter result may seem at variance with prior cross-sectional studies [4], it may be argued that the mean and the variance of HbA1c in the present sample were relatively low, possibly attenuating existing correlations.
A cross-sectional study does not allow causal inferences. Glucose fluctuations could facilitate the development of PD, and PD-induced systemic inflammation could affect glucose control [16]. Notably, in multivariate analyses the association of mean CAL and glucose CV was significant in models in which either mean CAL or glucose CV were imputed as dependent variable, supporting the possibility of a bi-directional association [16]. Causal relationships can be explored only through interventional studies. In one small trial in type 2 diabetes, the improvement of glycemic control produced marginal benefits on PD [17]. On the other hand, a Cochrane Review on the treatment of PD in mostly type 2 diabetic patients, including 35 randomized trials, showed a low-quality evidence of a modest short-term improvement of HbA1c [5].
No specific data on the effect of PD treatment on glucose variability have been reported so far.
Some limitations of the present study should be recognized. The sample size, although deemed sufficient for the principal endpoint with a formal power calculation, is relatively small. In addition, the sample cannot be considered representative of the whole population of patients with type 1 diabetes. On the other hand, the study has some strengths, such as the accuracy in the assessment of PD, and the availability of glucose monitoring data from all patients.
In conclusion, periodontal disease is associated with glucose variability in patients with type 1 diabetes. Further, interventional studies are needed to verify the effect of reduction of glucose variability on the evolution of periodontal disease, and those of periodontal treatment on glucose variability.
References
Sanz M, Quirynen M, European Workshop in Periodontology group (2005) Advances in the aetiology of periodontitis. Group a consensus report of the 5th european workshop in periodontology. J Clin Periodontol 32(6):54–56
Kinane DF, Preshaw PM, Loos BG (2011) Host-response: understanding the cellular and molecular mechanisms of host-microbial interactions—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38(suppl 11):44–48
Kassebaum NJ, Bernabé E, Dahiya M, et al. (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 93(11):1045–1053
Dicembrini I, Serni L, Monami MC et al (2020) Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol 57(12):1405–1412
Simpson TC, Weldon JC, Worthington HV et al (2005) Treatment of periodontal disease for glycaemic control in people with diabetes mellitus (2015). Cochrane Database Syst Rev 2015(11):CD004714
Preshaw PM, Alba AL, Herrera D, et al. (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55:21–31
Mealey BL, Ocampo GL (2007) Diabetes mellitus and periodontal disease Periodontol 44:127–153
Demmer RT, Desvarieux M, Holtfreter B, et al. (2010) Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care 33:1037–1043
Taylor JJ, Preshaw PM, Lalla E (2013) A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol 40(Suppl 14):S113–S134
Kovatchev B (2017) Metrics for glycaemic control — from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 13:425–436
Papapanou PN, Sanz M, Buduneli N, et al. (2018) Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 45(Suppl 20):S162–S170
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol 45(Suppl 20):S149–S161
Dakovic D, Pavlovic MD (2008) Periodontal disease in children and adolescents with type 1 diabetes in Serbia. J Periodontol 79(6):987–992. https://doi.org/10.1902/jop.2008.070549
Popławska-Kita A, Siewko K, Szpak P, et al. (2014) Association between type 1 diabetes and periodontal health. Adv Med Sci 59(1):126–131
Hodge PJ, Robertson D, Paterson K, et al. (2012) Periodontitis in non-smoking type 1 diabetic adults: a cross-sectional study. J Clin Periodontol 39(1):20–29
Bissett SM, Rapley T, Preshaw PM, et al. (2020) Uptake of best practice recommendations in the management of patients with diabetes and periodontitis: a cross-sectional survey of healthcare professionals in primary care. BMJ Open 10(1):e032369
Sanz M, Ceriello A, Buysschaert M, et al. (2018) Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 45(2):138–149
Katagiri S, Nitta H, Nagasawa T et al (2013) Effect of glycemic control on periodontitis in type 2 diabetic patients with periodontal disease. J Diabetes Investig 4(3):320–325
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
ID, FC, and EM contributed to the conception, design, enrollment, clinical management of patients, acquisition of data and their analysis, drafted the manuscript, and provided final approval of the version to be published. LB, LS, LP, and MC contributed to enrollment, clinical management of patients, acquisition of data, and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Ilaria Dicembrini has received speaking fees from Merck, Novartis, Astra Zeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Novonordisk, Sanofi, and Novartis. Luigi Barbato, Lapo Serni, Mariasmeralda Caliri, and Laura Pala have no conflicts of interest to declare. Francesco Cairo has received speaking fees from Straumann and Geistlich Biomaterials. Edoardo Mannucci has received consultancy fees from Merck and Novartis speaking fees from Astra Zeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Merck, Novonordisk, Sanofi, and Novartis and research grants from Merck, Novartis, and Takeda.
Ethical approval
The cross-sectional study was reported in guidance by STROBE guideline. The trial was approved by the Florence Ethical Board (approval number CEAVC 15,563/OSS). All analyses were performed using SPSS 16.0.
Informed consent
Informed consent was obtained from all study participants.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dicembrini, I., Barbato, L., Serni, L. et al. Glucose variability and periodontal disease in type 1 diabetes: a cross-sectional study—The “PAROdontopatia e DIAbete” (PARODIA) project. Acta Diabetol 58, 1367–1371 (2021). https://doi.org/10.1007/s00592-021-01720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01720-y