Abstract
Aims
To compare the long-term functional and anatomical outcomes of cataract surgery with combined versus 1-month deferred intravitreal dexamethasone implant (DEX) in eyes with pre-existing diabetic macular edema (DME).
Methods
Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were retrospectively evaluated in both groups before treatments, then 1, 4, 12 and 24 months after DEX.
Results
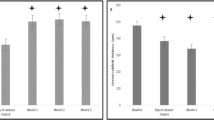
Forty eyes were analyzed, 20 in each group. BCVA disclosed comparable trends, increasing from similar starting values (p = 0.9913) to akin scores 1 month after DEX (p = 0.4229). After 4 months, it similarly reduced without significant variations within each group throughout the whole observation period. CRT was similar at the time of surgery (p = 0.6134) and was reduced by DEX injection in both samples, with a superior beneficial effect in the combined group after 1 month (p = 0.0010). At 4 months, CRT further elevated and remained overall stable in the long term without differences. By 12 months, 19 (95%) eyes received further injections: 1 (5%) fluocinolone, 3 (15%) received other DEX and fluocinolone, 13 (65%) ≥ 1 DEX only and 2 (10%) anti-VEGFs. During the second year, 6 additional eyes (from the 13 receiving DEX) switched to fluocinolone, reaching a total of 10 (50%). Similar results were observed in the deferred group.
Conclusions
DEX implant performed at the time of surgery achieved the same long-term functional and anatomical outcomes compared to a 1-month injection deferral in treating eyes with pre-existing DME that should undergo cataract extraction.



Similar content being viewed by others
References
Panozzo G, Staurenghi G, Dalla Mura G et al (2019) Prevalence of diabetes and diabetic macular edema in patients undergoing senile cataract surgery in Italy: the DIabetes and CATaract study. Eur J Ophthalmol. https://doi.org/10.1177/1120672119830578
Hayashi K, Igarashi C, Hirata A, Hayashi H (2009) Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 23:389–396. https://doi.org/10.1038/sj.eye.6703022
Sarao V, Veritti D, Maurutto E et al (2018) Pharmacotherapeutic management of macular edema in diabetic subjects undergoing cataract surgery. Expert Opin Pharmacother 19:1551–1563
Chu CJ, Johnston RL, Buscombe C et al (2016) Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology 123:316–323. https://doi.org/10.1016/j.ophtha.2015.10.001
Denniston AK, Chakravarthy U, Zhu H et al (2017) The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group, Report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol 101:1673–1678. https://doi.org/10.1136/bjophthalmol-2016-309838
Dowler JG, Sehmi KS, Hykin PG, Hamilton AM (1999) The natural history of macular edema after cataract surgery in diabetes. Ophthalmology 106:663–668. https://doi.org/10.1016/S0161-6420(99)90148-3
Il KS, Hwang DJ, Seo JY, Park IW (2011) Evaluation of changes of macular thickness in diabetic retinopathy after cataract surgery. Korean J Ophthalmol 25:238–242. https://doi.org/10.3341/kjo.2011.25.4.238
Chen C-H, Liu Y-C, Wu P-C (2009) The combination of intravitreal bevacizumab and phacoemulsification surgery in patients with cataract and coexisting diabetic macular edema. J Ocul Pharmacol Ther 25:83–89. https://doi.org/10.1089/jop.2008.0068
Akinci A, Muftuoglu O, Altınsoy A, Ozkılıc E (2011) Phacoemulsification with intravitreal bevacizumab and triamcinolone acetonide injection in diabetic patients with clinically significant macular edema and cataract. Retina 31:755–758. https://doi.org/10.1097/IAE.0b013e3182006da1
Rauen PI, Ribeiro JAS, Almeida FPP et al (2012) Intravitreal injection of ranibizumab during cataract surgery in patients with diabetic macular edema. Retina 32:1799–1803. https://doi.org/10.1097/IAE.0b013e31824bebb8
Lam DSC, Chan CKM, Mohamed S et al (2005) Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular oedema: a 6-month prospective pilot study. Eye (Lond) 19:885–890. https://doi.org/10.1038/sj.eye.6701686
Habib MS, Cannon PS, Steel DHW (2005) The combination of intravitreal triamcinolone and phacoemulsification surgery in patients with diabeticfoveal oedema and cataract. BMC Ophthalmol 5:15. https://doi.org/10.1186/1471-2415-5-15
Nunome T, Sugimoto M, Kondo M, Suto C (2018) Short-term results of intravitreal triamcinolone acetonide combined with cataract surgery for diabetic macular edema in japan: in the era of anti-vascular endothelial growth factor therapy. Ophthalmologica 240:73–80. https://doi.org/10.1159/000487548
Lim LL, Morrison JL, Constantinou M et al (2016) Diabetic macular edema at the time of cataract surgery trial: a prospective, randomized clinical trial of intravitreous bevacizumab versus triamcinolone in patients with diabetic macular oedema at the time of cataract surgery—preliminary 6 month results. Clin Exp Ophthalmol 44:233–242. https://doi.org/10.1111/ceo.12720
Ozgur OR, Ozkurt Y, Kulekci Z, Evciman T (2016) The combination of phacoemulsification surgery and intravitreal triamcinolone injection in patients with cataract and diabetic macular edema. Saudi J Ophthalmol Off J Saudi Ophthalmol Soc 30:33–38. https://doi.org/10.1016/j.sjopt.2015.10.004
Murtha T, Cavallerano J (2007) The management of diabetic eye disease in the setting of cataract surgery. Curr Opin Ophthalmol 18:13–18. https://doi.org/10.1097/ICU.0b013e32801129fc
Fraser-Bell S, Kaines A, Hykin PG (2008) Update on treatments for diabetic macular edema. Curr Opin Ophthalmol 19:185–189
Lattanzio R, Cicinelli MV, Bandello F (2017) Intravitreal steroids in diabetic macular edema. Dev Ophthalmol 60:78–90. https://doi.org/10.1159/000459691
Sacconi R, Giuffrè C, Corbelli E et al (2019) Emerging therapies in the management of macular edema: a review [version 1; peer review: 2 approved]. F1000 Research Ltd, London
Sacconi R, Parodi MB, Casati S et al (2017) Dexamethasone implants in diabetic macular edema patients with high visual acuity. Ophthalmic Res 58:125–130. https://doi.org/10.1159/000477256
Sacconi R, Corbelli E, Carnevali A et al (2018) Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol 102:1684–1690. https://doi.org/10.1136/bjophthalmol-2017-311240
Sze AM, Luk FO, Yip TP et al (2015) Use of intravitreal dexamethasone implant in patients with cataract and macular edema undergoing phacoemulsification. Eur J Ophthalmol 25:168–172. https://doi.org/10.5301/ejo.5000523
Agarwal A, Gupta V, Ram J, Gupta A (2013) Dexamethasone intravitreal implant during phacoemulsification. Ophthalmology 120(211):211.e1–5. https://doi.org/10.1016/j.ophtha.2012.08.002
Furino C, Boscia F, Niro A et al (2017) Combined phacoemulsification and intravitreal dexamethasone implant (Ozurdex®) in diabetic patients with coexisting cataract and diabetic macular edema. J Ophthalmol 2017:4896036. https://doi.org/10.1155/2017/4896036
Panozzo GA, Gusson E, Panozzo G, Dalla Mura G (2017) Dexamethasone intravitreal implant at the time of cataract surgery in eyes with diabetic macular edema. Eur J Ophthalmol 27:433–437. https://doi.org/10.5301/ejo.5000920
Panozzo G, Cicinelli MV, Augustin AJ et al (2020) An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol 30:8–18. https://doi.org/10.1177/1120672119880394
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Corbelli, Dr. Fasce, Dr. Iuliano, Dr. Sacconi and Dr. Lattanzio have nothing to disclose. Dr. Bandello reports personal fees from Alcon (Fort Worth, Texas, USA), personal fees from Alimera Sciences (Alpharetta, Georgia, USA), personal fees from Allergan Inc. (Irvine, California, USA), personal fees from Farmila-Thea (Clermont-Ferrand, France), personal fees from Bayer Schering Pharma (Berlin, Germany), personal fees from Bausch And Lomb (Rochester, New York, USA), personal fees from Genentech (San Francisco, California, USA), personal fees from Hoffmann-La-Roche (Basel, Switzerland), personal fees from Novagali Pharma (Évry, France), personal fees from Novartis (Basel, Switzerland), personal fees from Sanofi-Aventis (Paris, France), personal fees from ThromboGenics (Heverlee, Belgium), personal fees from Zeiss (Dublin, USA), outside the submitted work. Dr. Querques reports personal fees from Alimera Sciences (Alpharetta, Georgia, USA), personal fees from Allergan Inc. (Irvine, California, USA), personal fees from Amgen (Thousand Oaks, USA), personal fees from Heidelberg (Germany), personal fees from KBH (Chengdu, China), personal fees from LEH Pharma (London, UK), personal fees from Lumithera (Poulsbo, USA), personal fees from Novartis (Basel, Switzerland), personal fees from Bayer Schering Pharma (Berlin, Germany), personal fees from Sandoz (Berlin, Germany), personal fees from Sifi (Catania, Italy), personal fees from Soof-Fidia (Albano, Italy), personal fees from Zeiss (Dublin, USA), outside the submitted work.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Corbelli, E., Fasce, F., Iuliano, L. et al. Cataract surgery with combined versus deferred intravitreal dexamethasone implant for diabetic macular edema: long-term outcomes from a real-world setting. Acta Diabetol 57, 1193–1201 (2020). https://doi.org/10.1007/s00592-020-01509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01509-5