Abstract
Background and aims
One cohort and several basic science studies have raised suspicion about an association between incretin therapies and cholangiocarcinoma. Our aim was to verify the occurrence of CC in relation to incretin-based medication use versus any antidiabetic treatment in an unselected population of diabetic patients.
Methods
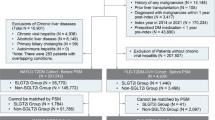
A population-based matched case–control study was conducted using administrative data from the Region of Piedmont (4,400,000 inhabitants), Italy. From a database of 312,323 patients treated with antidiabetic drugs, we identified 744 cases hospitalized for cholangiocarcinoma from 2010 to 2016 and 2976 controls matched for gender, age and initiation of antidiabetic therapy; cases and controls were compared for exposure to incretin-based medications. All analyses were adjusted for risk factors for CC, as ascertained by hospital discharge records. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by fitting a conditional logistic model.
Results
The mean age of the sampled population (cases and controls, 75 years) was very high, with no gender prevalence. Five per cent was treated with incretin-based medications. After adjusting for possible confounders, we found no increased risk of cholangiocarcinoma associated with the use of either DPP4i (OR 0.98, 95% CI 0.75–1.29: p = 0.89) or GLP-1-RA (OR 1.09, 95% CI 0.63–1.89; p = 0.76) in the 24 months before hospital admission. Neither the duration of the therapy nor the dose modified the risk of cholangiocarcinoma.
Conclusions
Our findings suggest that, in an unselected population, the use of both classes of incretin-based medications is not associated with an increased risk of cholangiocarcinoma.

Similar content being viewed by others
References
Razumilava N, Gores GJ (2014) Cholangiocarcinoma. Lancet 383(9935):2168–2179. https://doi.org/10.1016/s0140-6736(13)61903-0
Saengboonmee C, Seubwai W, Wongkham C, Wongkham S (2015) Diabetes mellitus: possible risk and promoting factors of cholangiocarcinoma: association of diabetes mellitus and cholangiocarcinoma. Cancer Epidemiol 39(3):274–278. https://doi.org/10.1016/j.canep.2015.04.002
Abrahami D, Douros A, Yin H, Yu OH, Faillie JL, Montastruc F et al (2018) Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: population based cohort study. BMJ 363:k4880. https://doi.org/10.1136/bmj.k4880
Marzioni M, Alpini G, Saccomanno S et al (2007) Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 133:244–255. https://doi.org/10.1053/j.gastro.2007.04.007
Chen BD, Zhao WC, Dong JD, Sima H (2014) Expression of GLP-1R protein and its clinical role in intrahepatic cholangiocarcinoma tissues. Mol Biol Rep 41:4313–4320. https://doi.org/10.1007/s11033-014-3302-7
Marzioni M, Alpini G, Saccomanno S et al (2009) Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut 58:990–997. https://doi.org/10.1136/gut.2008.150870
Han Y, Glaser S, Meng F, Francis H, Marchioni M, McDaniel K et al (2013) Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp Biol Med (Maywood) 238(5):549–565. https://doi.org/10.1177/1535370213489926
Davies MJ, D’Alessio DA, Fradkin J et al (2018) Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12):2669–2701. https://doi.org/10.2337/dci18-0033
Noel RA, Braun DK, Patterson RE, Bloomgren GL (2009) Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 32:834–838
Lando HM, Alattar M, Dua AP (2012) Elevated amylase and lipase levels in patients using glucagon-like peptide-1 receptor agonists or dipeptidyful-peptidase-4 inhibitors in the outpatient setting. Endocr Pract 18:472–477
Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L (2016) Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med 176(10):1474–1481. https://doi.org/10.1001/jamainternmed.2016.1531
Leiter LA, Teoh H, Mosenzon O et al (2016) SAVOR-TIMI 53 steering committee and investigators. Frequency of cancer events with saxagliptin in the SAVOR-TIMI 53 trial. Diabetes Obes Metab 18:186–190. https://doi.org/10.1111/dom.12582
Marso SP, Daniels GH, Brown-Frandsen K et al (2016) LEADER steering committee, LEADER trial investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375:311–322. https://doi.org/10.1056/nejmoa1603827
Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB (2018) LEADER Publication Committee on behalf of the LEADER trial investigators. Neoplasms reported with liraglutide or placebo in people with type diabetes: results from the LEADER randomized trial. Diabetes Care 41:1663–1671. https://doi.org/10.2337/dc17-1825
Suissa S, Dell’Aniello S, Vahey S, Renoux C (2011) Time-window bias in case–control studies—statins and lung cancer. Epidemiology 22:228–231
Piccinelli C, Carnà P, Stringhini S et al (2018) The contribution of behavioural and metabolic risk factors to socioeconomic inequalities in mortality: the Italian Longitudinal Study. Int J Public Health 63:325–335
Author information
Authors and Affiliations
Contributions
CBG and RG performed literature search, study design, data collection, data interpretation and writing; RP was involved in data collection and data analysis; EN and BT carried out literature search and data collection; LM contributed to literature search, study design and data interpretation; GC performed data interpretation and writing; and CBG, RP, GC and RG had access to the raw data. CBG had full access to all the data in the study and the final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors have nothing to disclose.
Ethical Standard Statement
This is an observational study and data were collected retrospectively. The Local Health was responsible for collecting and processing these data. The study was performed in accordance with Local Health Authority. No ethical approval was required according to Italian Law 211/2003, which explains that no ethics committee permission is required for this kind of study.
Informed consent
According to Italian Privacy Law, no patient’s or relative’s consent is required for large retrospective population-based studies if data are published only in aggregated form.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giorda, C.B., Picariello, R., Tartaglino, B. et al. Incretin-based therapy and risk of cholangiocarcinoma: a nested case–control study in a population of subjects with type 2 diabetes. Acta Diabetol 57, 401–408 (2020). https://doi.org/10.1007/s00592-019-01444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01444-0