Abstract
Aims
To assess the prospective association between baseline serum uric acid level and subsequent risk of development or progression in albuminuria.
Methods
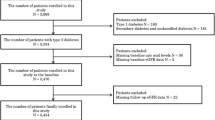
Longitudinal data were obtained from 2518 patients with type 2 diabetes in the development cohort and registered in a Japanese diabetes registry. To assess the independent correlations between baseline serum uric acid quartiles and either the development or progression of diabetic nephropathy for 2 years, the Cox proportional hazards model was used and adjusted for potential confounders.
Results
The mean patient age, body mass index, and glycated hemoglobin (HbA1c) level were 66.1 years, 24.6 kg/m2, and 7.5 % (57.6 mmol/mol), respectively. The baseline serum uric acid levels, with mean values of 3.6, 4.9, 5.8, and 7.3 mg/dL from the first to fourth quartiles, were significantly associated with the urinary albumin/creatinine ratio at baseline (p < 0.001). Baseline uric acid levels were not significantly associated with the development of nephropathy, but they were with the progression of nephropathy. The multivariable-adjusted hazards ratios for the progression from microalbuminuria to macroalbuminuria were 2.17 [95 % confidence interval (CI) 1.15–4.08; p = 0.016], 3.04 (95 % CI 1.67–5.53; p < 0.001), and 3.56 (95 % CI 1.83–6.93; p < 0.0011) for the first, third, and fourth quartiles of serum uric acid levels, respectively, as compared to that for the second quartile. We did not observe significant association between uric acid levels and change in estimated glomerular filtration rate.
Conclusions
Low and high serum uric levels, independent of possible confounders, were associated with a subsequent risk of progression, not development, in albuminuria in type 2 diabetes patients. Therefore, serum uric acid levels may be useful for predicting the future risk of progression of microalbuminuria.

Similar content being viewed by others
References
Ritz E, Orth SR (1999) Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 341:1127–1133
Caramori ML, Fioretto P, Mauer M (2000) The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 49:1399–1408
Gerstein HC et al (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286:421–426
Weir MR (2007) Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol 2:581–590
Rossing P (2006) Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia 49:11–19
Rossing P (2006) Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep 6:479–483
Mazzali M et al (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282:F991–F997
Domrongkitchaiporn S et al (2005) Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol 16:791–799
Iseki K et al (2004) Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 44:642–650
Ishani A et al (2006) Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17:1444–1452
Obermayr RP et al (2008) Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19:2407–2413
Ficociello LH et al (2010) High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 33:1337–1343
Fukui M et al (2008) Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism 57:625–629
Tseng CH (2005) Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int 68:796–801
Hayashino Y et al (2012) The association between problem areas in diabetes scale scores and glycemic control is modified by types of diabetes therapy: diabetes distress and care registry in Tenri (DDCRT 2). Diabetes Res Clin Pract 97:405–410
Tsujii S, Hayashino Y, Ishii H, Diabetes Distress and Care Registry at Tenri Study Group (2012) Diabetes distress, but not depressive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 1). Diabet Med 29:1451–1455
Mashitani T et al (2013) Patient-reported adherence to insulin regimen is associated with glycemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 3). Diabetes Res Clin Pract 100:189–194
Hayashino Y, Mashitani T, Tsujii S, Ishii H, Diabetes Distress and Care Registry at Tenri Study Group (2014) Elevated levels of hs-CRP are associated with high prevalence of depression in Japanese patients with type 2 diabetes: the Diabetes Distress and Care Registry at Tenri (DDCRT 6). Diabetes Care 37:2459–2465
Hayashino Y, Mashitani T, Tsujii S, Ishii H (2014) Serum high-sensitivity C-reactive protein levels are associated with high risk of development, not progression, of diabetic nephropathy among Japanese Type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT7]). Diabetes Care 37:2947–2952
Mashitani T, Hayashino Y, Okamura S, Tsujii S, Ishii H (2014) Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT 5]). Diabetes Care 37:252–258
Hayashino Y, Tsujii S, Ishii H, Diabetes Distress and Care Registry at Tenri Study Group (2013) High frequency of non-nocturnal hypoglycemia was associated with poor sleep quality measure by Pittsburg Sleep Quality Index in patients with diabetes receiving insulin therapy: Diabetes Distress and Care Registry at Tenri (DDCRT 4). Exp Clin Endocrinol Diabetes 121:628–634
The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 1:212–228 (2010)
Matsuo S et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Molitch ME et al (2004) Nephropathy in diabetes. Diabetes Care 27(Suppl 1):S79–S83
Wada T et al (2014) Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol 18:613–620
Hess KR (1995) Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 14:1707–1723
Brown ER, Ibrahim JG, DeGruttola V (2005) A flexible B-spline model for multiple longitudinal biomarkers and survival. Biometrics 61:64–73
Heinzl H, Kaider A (1997) Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 54:201–208
Johnson RJ et al (2003) Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41:1183–1190
Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB (1999) Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis 33:225–234
Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G (2001) Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31:318–321
Mene P, Punzo G (2008) Uric acid: bystander or culprit in hypertension and progressive renal disease? J Hypertens 26:2085–2092
Sanchez-Lozada LG et al (2005) Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 67:237–247
Khosla UM et al (2005) Hyperuricemia induces endothelial dysfunction. Kidney Int 67:1739–1742
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Rosolowsky ET et al (2008) High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 3:706–713
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009) Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668–1671
Jalal DI et al (2010) Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant 25:1865–1869
Tanaka K et al (2015) Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J Diabetes Investig 6:98–104
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78:6858–6862
Pan HZ et al (2010) The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 47(Suppl 1):71–76
Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19:257–267
Lee SM et al (2009) Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol 29:79–85
Siu YP, Leung KT, Tong MK, Kwan TH (2006) Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47:51–59
Dosemeci M, Wacholder S, Lubin JH (1990) Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol 132:746–748
Acknowledgments
This study was partially supported by the Manpei Suzuki Diabetes Foundation and JSPS KAKENHI (Grant No. 25460641); however, they played no role in the study design or conduct, data collection, analysis, or interpretation, and the preparation, review, or approval of the manuscript. We would like to especially thank Yukari Moritsuji, Yuki Fujita, Noriko Nakamura, and Yoko Sakamoto (Department of Endocrinology, Tenri Hospital) for their clerical support. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Okamura reports personal fees from Takeda Pharmaceutical Company, Ltd., personal fees from Daiichi Sankyo Company, Ltd., personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Kissei Pharmaceutical Co., Ltd., outside the submitted work. Dr. Hayashino reports personal fees from Takeda Pharmaceutical Company, Ltd., personal fees from Eli Lilly Japan K.K., personal fees from Daiichi Sankyo Company, Ltd., personal fees from Pfizer Japan Inc, personal fees from Ono Pharmaceutical Co., Ltd., outside the submitted work. Dr. Tsujii reports personal fees from AstraZeneca K.K., personal fees from Daiichi Sankyo Company, Ltd., personal fees from Eli Lilly Japan K.K., personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Novo Nordisk Pharma Ltd., personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Sanofi K.K., outside the submitted work. Dr. Ishii reports personal fees from Takeda Pharmaceutical Company, Ltd., personal fees from Eli Lilly Japan K.K., personal fees from Sanofi KK., personal fees from Merck & Co., Inc., personal fees from Astellas Pharma Inc., personal fees from Astellas Pharma Inc., personal fees from Novartis Pharma K.K., personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Daiichi Sankyo Company, Ltd., personal fees from Ono Pharmaceutical Co., Ltd, personal fees from AstraZeneca K.K., personal fees from Taisho Toyama Pharmaceutical Co., Ltd, personal fees from SHIONOGI & CO., LTD., personal fees from Kowa Pharmaceutical Co., Ltd, personal fees from Boehringer Ingelheim, outside the submitted work.
Ethical standard
The study was approved by the Ethical Committee of Tenri Hospital, Japan.
Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Porta.
For the Diabetes Distress and Care Registry at Tenri Study Group.
Members of the Diabetes Distress and Care Registry at Tenri Study Group are listed in the Appendix.
Appendix: Members of the Diabetes Distress and Care Registry at Tenri Study Group
Appendix: Members of the Diabetes Distress and Care Registry at Tenri Study Group
Shintaro Okamura, Miyuki Furuya, Masako Kitatani, Hirohito Kuwata (Department of Endocrinology, Tenri Hospital); Satoru Tsujii (Diabetes Center, Tenri Hospital); Yasuaki Hayashino (Department of Endocrinology, Tenri Hospital, Department of Epidemiology and Healthcare Research, Kyoto University Graduate School of Medicine); Hitoshi Ishii, Tsuyoshi Mashitani (Nara Medical University); Satoshi Matsunaga (Department of Hematology, Endocrinology and Metabolism, Niigata University Faculty of Medicine); Yaeko Kondo, Naotaka Fujita (Department of Diabetes and Clinical Nutrition, Kyoto University); Rei Ueda (Second Department of Internal Medicine, Faculty of Medicine, University of the Ryukyus); Rie Kurokawa (Osaka Medical Center and Research Institute for Maternal and Child Health); Masami Tanaka (Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, Keio University School of Medicine).
Rights and permissions
About this article
Cite this article
Hayashino, Y., Okamura, S., Tsujii, S. et al. Association of serum uric acid levels with the risk of development or progression of albuminuria among Japanese patients with type 2 diabetes: a prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 10)]. Acta Diabetol 53, 599–607 (2016). https://doi.org/10.1007/s00592-015-0825-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0825-x