Abstract
Aims
We have previously described the insulinotropic activities of [I10W]tigerinin-1R (RVCSAIPLPWCH.NH2) in vitro. In this study, we investigated the effects of the peptide on nutrient homoeostasis in mice with diet-induced obesity and insulin resistance.
Methods
Male NIH Swiss mice were maintained on a high-fat diet for 12 weeks prior to the study. Twice-daily intraperitoneal injections of [I10W]tigerinin-1R (75 nmol/kg body weight) were administered for 28 days. Body weight, energy intake, body fat content, and plasma concentrations of triglyceride, cholesterol, non-fasting glucose and insulin were monitored. Effects of the peptide on glycaemic control were measured by glucose tolerance and insulin sensitivity tests. Pancreatic hormone content and insulin secretory responses of islets isolated from treated and untreated mice were examined. Immunohistochemical analysis was performed to study possible changes in islet morphology.
Results
Administration of [I10W]tigerinin-1R to high-fat-fed mice produced significant (P < 0.05) decreases in plasma glucose, glucagon and triglyceride concentrations and an increase in plasma insulin compared to high-fat-fed controls. No changes in body weight or energy intake were observed with peptide treatment, but glycaemic control was significantly improved in response to oral or intraperitoneal glucose. Insulin sensitivity and secretory responses of islets to established insulin secretagogues were also significantly improved in peptide-treated mice. Total body fat, pancreatic insulin and glucagon contents, islet, beta and alpha cell areas were all significantly decreased in treated mice.
Conclusions
This study shows that [I10W]tigerinin-1R improves insulin sensitivity, islet function and glycaemic control in high-fat-fed mice and has potential as a template for development of novel anti-diabetic agents.
Similar content being viewed by others
Introduction
The current pandemic of type 2 diabetes mellitus (T2DM) has stimulated a search for natural products with therapeutic potential. Several peptides isolated from the venoms of a range of vertebrate and invertebrate species have been shown to possess insulinotropic activities. For example, mastoparan, a peptide from wasp venom, potentiates insulin release from βTC3 and INS-1 cells [1] and venom of the snake, Walterinnesia aegyptia decreases blood glucose levels in dogs [2]. Vm24, a 36 amino acid peptide isolated from venom of scorpion Vaejovis mexicanus, inhibits the proliferation and signalling of T cells and so has potential to be used to treat autoimmune disorders including type 1 diabetes mellitus (T1DM) [3]. Similarly, venom from the scorpion, Androctonus crassicauda has also been shown to have potential use for the treatment of T1DM [4]. Of particular importance is exendin-4, a 39 amino acid peptide isolated from saliva of Gila monster lizard, Heloderma suspectum that shares 53 % structural similarity with GLP-1 and mimics the actions of the peptide [5]. Exendin-4 increases intracellular cAMP levels and potentiates glucose-stimulated insulin secretion [6]. Exendin-4 exhibits higher metabolic stability and hence longer half-life than GLP-1, making it a good candidate for therapy of patients with T2DM. Synthetic exendin-4, under trade name Byetta, was approved for clinical use in 2005 and is used extensively in diabetes therapy [7].
The success of exendin-4 in diabetes treatment forms the basis for a comparative approach to identify potential anti-diabetic peptides from amphibian skin secretions. Several peptides isolated from frogs belonging to the Bombinatoridae, Dicroglossidae, Hylidae, Leptodactylidae, Pipidae, and Ranidae families stimulate insulin release from clonal BRIN-BD11 pancreatic β-cells and improve glucose tolerance in animal models of T2DM [8]. The tigerinins are small (11 or 12 amino acid residues), cationic (charge of +2 at pH 7), C-terminally α-amidated peptides that contain a single disulphide bridge. Peptides of the tigerinin family with antimicrobial activity were first identified in the skin of Indian frog Hoplobatrachus tigerinus (formerly classified as Rana tigerina) in the family Dicroglossidae [9] and subsequently in Fejervarya cancrivora [10] in the same family. The tigerinins are not confined to the Dicroglossidae as tigerinin-1V has been isolated from skin secretions of the Costa Rican frog, Lithobates vaillanti (Ranidae) [11] and tigerinin-1M from skin secretions of the east-African Mueller’s clawed frog Xenopus muelleri (Pipidae) [12]. Tigerinin-1R, lacking antimicrobial activities, was isolated from an extract of skin of the Vietnamese common lowland frog Hoplobatrachus rugulosus [13]. Tigerinin-1R is a potent stimulator of insulin release from BRIN-BD11 cells without cell toxicity, and the peptide is effective in vivo, increasing insulin concentration and improving glucose tolerance in acute [13] and longer-term [14] studies in animal models of obesity-diabetes [13]. Tigerinin-1R also stimulates the release of GLP-1 from GLP-1 secreting GLUTag cell lines [15] and increases production of the cytokines IL-6 and IL-10 by mouse peritoneal macrophages [16].
We recently reported the effects on in vitro insulin release and cytotoxicity of changes in cationicity and hydrophobicity of tigerinin-1R produced by substitutions of selected amino acids by l-arginine, l-lysine and l-tryptophan [17]. The [A5W], [L8W] and [I10W] analogues produce a significant (P < 0.01) increase in insulin release from BRIN-BD11 cells at concentration of 0.01 nM compared with 0.1 nM for tigerinin-1R. Insulin-releasing activity of the native tigerinin-1R and its substituted analogues was associated with membrane depolarisation and enhanced intracellular Ca2+ levels. In the light of these promising preliminary results, together with the observed metabolic effects of native tigerinin-1R [14], an investigation of the longer-term in vivo antidiabetic actions of the superior insulin-releasing novel analogue, [I10W]tigerinin-1R in an animal model of T2DM was carried out. The present study examines the biological effects of 28-day administration of [I10W]tigerinin-1R on glucose tolerance, insulin sensitivity, the function and morphology of pancreatic islets, energy expenditure, body composition, and plasma triglyceride and cholesterol concentrations in mice with established diet-induced obesity and insulin resistance.
Materials and methods
Peptide synthesis and purification
[I10W]tigerinin-1R (RVCSAIPLPWCH.NH2) was supplied in crude form by GL Biochem Ltd (Shanghai, China). Purification to near homogeneity (>98 % purity) was performed by reversed-phase HPLC on a (2.2 cm × 25 cm) Vydac 218TP1022 (C18) column equilibrated with acetonitrile/water/trifluoroacetic acid (21.0/78.9/0.1 v/v/v) at a flow rate of 6 ml/min. The concentration of acetonitrile was raised to 56 % (v/v) over 60 min. Identity of the peptide was confirmed by MALDI-TOF mass spectrometry.
Laboratory animals
National Institutes of Health (NIH) Swiss mice (male, 8 weeks old, Harlan Ltd, UK) were housed individually in an air-conditioned room (22 ± 2 °C) with a 12-h light/12-h dark cycle (08:00–20:00 h). Mice were maintained for 3 months prior to the experiment on a high-fat diet consisting of 45 % kcal from fat, 20 % kcal from protein, and 35 % kcal from carbohydrate (total energy 26.15 kJ/g, Dietex International Ltd, Witham, UK) or standard rodent diet. Weight-matched mice with clearly manifested obesity, insulin resistance and glucose intolerance together with their age matched lean controls were used in this study. All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63EU for animal experiments. No adverse effects were observed in mice treated with [I10W]tigerinin-1R.
In vivo experimental procedure
High-fat-fed mice (n = 8) received twice-daily intraperitoneal injections of either saline vehicle [0.9 % (w/v) or [I10W]tigerinin-1R (75 nmol/kg body weight)] for 28 days. This peptide dose was selected from results of a pilot study that investigated doses of 50, 75 and 150 nmol/kg body weight. Twice-daily injection of [I10W]tigerinin-1R was selected based on previous observations that the peptide is metabolically active within 12 h of administration. Energy intake, body weight, non-fasting blood glucose and plasma insulin concentrations were measured 3 days prior to and every 72 h during the study. Glucose tolerance (18 mmol/kg body weight, i.p. or oral) and insulin sensitivity tests (25 IU/kg bw) were carried out after 28 days as previously described [18]. HOMA IR was calculated using the formula: HOMA IR = fasting plasma insulin x fasting plasma glucose/22.5. Indirect calorimetry and energy expenditure in high-fat-fed mice were measured using the Comprehensive Laboratory Animal Monitoring System (CLAMS) metabolic chambers (Columbus Instruments, Columbus, OH, USA) [19]. Terminal analysis included measurement of total body lean and fat mass, bone mineral density and bone mineral content by DXA scanning (Piximus Densitometer, USA). Skeletal muscle was collected and stored for gene expression analysis [20]. Pancreatic tissue was excised for islet isolation, morphological evaluation and analysis of insulin and glucagon contents following homogenisation in extraction buffer (20 mM TrisHCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 0.5 % Triton X 100, pH 7.5) using a VWR VDI 12 handheld homogeniser (VWR, UK).
Insulin release from isolated islets
Insulin secretory function of islets isolated from mice receiving [I10W]tigerinin-1R or saline was assessed at 1.4 mM glucose and in response to established insulin secretagogues including incretin hormones: 16.7 mM glucose, 10 mM alanine, 10 mM arginine, 30 mM KCl, 1 µM GLP-1 and 1 µM GIP. The non-glucose secretagogues were tested in the presence of 16.7 mM glucose. Islets isolated by collagenase digestion [21] were cultured overnight in RPMI 1640 prior to a 40 min pre-incubation (5–10 islets per tube) at 37 °C in KRB buffer supplemented with 1.4 mM glucose. After this period, islets were incubated for 1 h in KRB buffer containing 1.4 mM glucose or 16.7 mM glucose and the agents indicated in Fig. 4. Insulin release was measured by radioimmunoassay as described previously [22], and results were expressed as a percentage of total insulin content of the islets.
Biochemical measurements
Blood samples (approximately 150 µl) were collected from the cut tip of the tail vein of unanaesthetised mice at intervals indicated in the figures, and plasma was separated and stored as described previously [13]. Blood glucose was measured using a hand-held Ascencia Contour meter (Bayer Healthcare, UK). Plasma and pancreatic insulin concentrations were determined by radioimmunoassay [22]. Enzyme-linked immunosorbent assay for measuring glucagon concentrations in plasma, and pancreatic extracts was carried out using a chemiluminescent ELISA kit (Millipore, MA, USA) following the manufacturer’s protocol. Plasma triglyceride and cholesterol concentrations were measured using an I-lab 650 clinical chemistry instrument (Instrumentation Laboratory, Warrington, UK) as previously described [23]. Total protein content in the extracts was measured using the Bradford reagent [24]. Expression in skeletal muscle of genes involved in insulin action, Insr and Slc2a4, was determined by polymerase chain reaction as outlined previously with mRNA expression normalised to Actb expression [20].
Immunohistochemical investigation of islet morphology
Pancreata were fixed in 4 % paraformaldehyde, processed using an automated tissue processor and embedded in paraffin. Sections (8 μM) were placed on slides, dewaxed and rehydrated. After blocking with 2 % BSA for 30 min, the slides were incubated with primary antibody [mouse anti-insulin antibody (1:500) and guinea pig anti-glucagon antibody (1:400)] overnight at 4 °C. Secondary antibody mixture (Alexa Fluor 594 goat anti-mouse antibody and Alexa Fluor 488 goat anti-guinea pig antibody) was added and incubated for 45 min at 37 °C. After staining the nucleus with 4′,6-diamidino-2-phenylindole (DAPI) stain, the slides were mounted using anti-fade mounting medium. The slides were then analysed using tetramethylrhodamine isothiocyanate filter (594 nm) or fluorescein isothiocyanate filter (488 nm) using a fluorescent microscope (Olympus System Microscope BX51, Olympus instruments, UK), and images were captured using DP70 camera adapter system. Cell^F imaging software (Olympus System Microscope BX51, Olympus instruments, UK) was used to determine islet number, islet area and both beta cell and alpha cell areas.
In vitro effects of [I10W]tigerinin-1R on beta cell growth
To evaluate possible direct effects of [I10W]tigerinin-1R on beta cell growth and viability, BRIN-BD11 cells were seeded at a density of 50,000 cells per well and grown in RPMI 1640 tissue culture medium supplemented with 10 % (v/v) foetal calf serum, antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin) and 11.1 ml glucose) at 37 °C in an atmosphere of 5 % CO2 and 95 % air. Following a 12 h incubation to allow cells attach, the incubation medium was replaced with fresh control medium or medium supplemented with streptozotocin (5 mmol/l, positive control) or graded doses of [I10W]tigerinin-1R (0.01–1 μM). After a further 24 h, cells were detached from the plates using trypsin/EDTA and the number of viable cells was determined after trypan blue staining using haemocytometer and an Olympus CKX41 inverted microscope. Cell number increased by 2.1-fold over 24 h. Incubation of cells with streptozotocin caused 62 % decrease in cell number (P < 0.001) compared with control. [I10W]tigerinin-1R (0.01–1 μM) had no effect of growth of beta cells, ruling out possible direct effects on viability, beta cell proliferation/apoptosis.
Statistical analysis
Results are expressed as mean ± SEM. Values are compared using one-way ANOVA followed by Student–Newman–Keuls post hoc test. Area under the curve (AUC) analysis was performed using the trapezoidal rule with baseline correction. P < 0.05 was considered statistically significant.
Results
Effects of [I10W]tigerinin-1R on energy intake, body weight, non-fasting blood glucose and plasma insulin
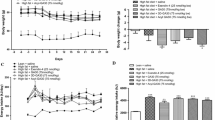
Body weight, energy intake and non-fasting plasma glucose and insulin concentrations of high-fat-fed mice were significantly (P < 0.001) greater than that of lean control animals (Fig. 1). Twice-daily intraperitoneal administration of [I10W]tigerinin-1R (75 nmol/kg body weight) to mice on high-fat diet did not produce any significant changes in body weight and energy intake compared to mice administered with saline (Fig. 1a, b). A significant decrease (P < 0.05) in plasma glucose concentrations was observed from day 12 of treatment with the peptide, compared with high-fat-fed, saline controls (Fig. 1c). Plasma insulin concentrations were also significantly increased (P < 0.05) from day 18 of treatment, compared with high-fat-fed, saline controls (Fig. 1d).
Effects of [I10W]tigerinin-1R on body weight (a), cumulative energy intake (b), non-fasting plasma glucose (c) and insulin (d) in normal and high-fat-fed mice. Parameters were measured 3 days prior to, and every 72 h during twice-daily treatment (indicated by the black bar) with saline or [I10W]tigerinin-1R (75 nmol/kg bodyweight) for 28 days. Values are mean ± SEM for 8 mice. *P < 0.05 and ***P < 0.001 compared to high-fat-fed control
Effects of [I10W]tigerinin-1R on glucose tolerance and insulin secretion
A significant decrease (P < 0.05) in plasma glucose concentration was observed 60 min after i.p administration of glucose (18 mmol/kg body weight) in mice treated with [I10W]tigerinin-1R compared to high-fat-fed, saline controls (Fig. 2a). Integrated response over a period of 60 min, expressed as AUC (Fig. 2b), showed that glucose levels decreased (P < 0.05) by 30 % in animals treated with the peptide compared to animals treated with saline. A significant increase (P < 0.01) in plasma insulin concentration was observed 15 min after an intraperitoneal glucose load in mice treated with [I10W]tigerinin-1R compared to high-fat-fed, saline controls (Fig. 2c). The integrated response over a period of 60 min was significantly greater than that observed in high-fat-fed, saline controls (27 % increase in plasma insulin compared to high-fat-fed controls, P < 0.001, Fig. 2d).
Effects of [I10W]tigerinin-1R on plasma glucose and insulin concentrations following intraperitoneal (a–d) and oral (e–h) glucose administration (18 mmol/kg body weight) in normal and high-fat-fed mice. Tests were conducted after twice-daily treatment of mice with [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days. Values are mean ± SEM for 8 mice. **P < 0.01 and ***P < 0.001 compared with saline-treated lean mice. + P < 0.05 and +++ P < 0.001 compared to high-fat-fed control. Δ P < 0.05, ΔΔ P < 0.01, ΔΔΔ P < 0.001 compared with high-fat-fed control at the corresponding time point
In a second set of experiments, glucose (18 mmol/kg body weight) was administered orally to animals pre-treated for 28 days with peptide or saline. A significant decrease (P < 0.05) in plasma glucose concentrations was observed after 60 min in animals treated with [I10W]tigerinin-1R, compared with control animals treated with saline (Fig. 2e). The integrated response over a period of 60 min (expressed as AUC, Fig. 2f) was significantly lower than that of the control group (24 % decrease in plasma glucose concentration, P < 0.05). A significant increase (P < 0.05) in insulin concentration was observed at time points 15 and 30 min after glucose administration in animals treated with the peptide, compared to high-fat-fed, saline controls (Fig. 2g). The integrated response over a period of 60 min in peptide-treated animals was significantly greater than that in control animals (32 % increase in insulin concentration, P < 0.001, Fig. 2h).
Effects of [I10W]tigerinin-1R on insulin sensitivity
Insulin sensitivity of high-fat-fed mice treated with [I10W]tigerinin-1R showed significant improvement compared to high-fat-fed controls (Fig. 3). Following intraperitoneal administration of insulin (25 IU/kg body weight), plasma glucose concentration in animals treated with [I10W]tigerinin-1R was reduced by 39 % after 30 min and by 49 % after 60 min, compared to 26 % decrease after 30 min and 31 % decrease after 60 min observed in high-fat-fed controls treated with saline (Fig. 3a). Although the difference in plasma glucose concentration was not significant at any given time point compared to high-fat-fed controls, the integrated response over a period of 60 min in treated animals showed a significant decrease (P < 0.05) compared to controls (Fig. 3b). A significant impairment (P < 0.001) of insulin sensitivity was observed in mice fed a high-fat diet compared to normal controls as shown by HOMA IR (Fig. 3c). Treatment with [I10W]tigerinin-1R improved insulin sensitivity in mice fed a high-fat diet as assessed by HOMA IR (32 % decrease compared to high-fat-fed controls, P < 0.05). Feeding high-fat diet resulted in downregulation of the expression of Insr (20 %, P < 0.01) and Slc2a4 (54 %, P < 0.01) in skeletal muscle. Consistent with improved insulin sensitivity, no change was observed in [I10W]tigerinin-1R-treated mice.
Effects of [I10W]tigerinin-1R on insulin sensitivity (a–c), non-fasting plasma glucagon (d) and pancreatic glucagon (e) in normal and high-fat-fed mice. Plasma glucose concentrations were measured prior to, and after, intraperitoneal injection of insulin (25 IU/kg bw) in normal and high-fat-fed mice treated with twice-daily injections of saline or [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days. Plasma glucagon was measured at time zero. Blood from fasting animals was collected for HOMA IR. Values are mean ± SEM for 8 mice. **P < 0.01, ***P < 0.001 compared to saline-treated lean mice. Δ P < 0.05, ΔΔ P < 0.01 compared to high-fat-fed control
Effects of [I10W]tigerinin-1R on plasma glucagon concentrations
A significant increase (175 %, P < 0.01) in plasma glucagon concentrations in mice fed a high-fat diet was observed compared with normal mice. Treatment with [I10W]tigerinin-1R decreased the plasma glucagon concentration in high-fat-fed mice (42 % compared with high-fat-fed controls, P < 0.01) (Fig. 3d). Similar changes were observed for pancreatic glucagon content (Fig. 3e).
Effects of [I10W]tigerinin-1R on islet secretory responses
Insulin secretory responses of islets isolated from [I10W]tigerinin-1R-treated mice to 16.7 mM glucose, GLP-1, GIP and other established insulin secretagogues including alanine, l-arginine and KCl were significantly improved compared with high-fat-fed controls (Fig. 4a, b). This latter group exhibited impaired insulin secretory responses compared with mice fed normal diet, whereas high-fat-fed mice treated with [I10W]tigerinin-1R did not (Fig. 4a, b).
Insulin secretory responses of isolated islets (a, b), pancreatic weight (c) and pancreatic insulin content (d) of high-fat-fed mice treated with saline or [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days. Values are mean ± SEM for 8 mice. In b, stimulation index refers to the fold increase in insulin release compared to 1.4 mM glucose for 16.7 mM glucose control and compared to 16.7 mM glucose for all other secretagogues. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to 16.7 mM glucose. Δ P < 0.05, ΔΔ P < 0.01 and ΔΔΔ P < 0.001 compared to normal mice. + P < 0.05, ++ P < 0.01 and +++ P < 0.001 compared to high-fat-fed mice. For d, ***P < 0.001 compared with saline-treated lean mice. Δ P < 0.05 compared with high-fat-fed control
Effects of [I10W]tigerinin-1R on pancreatic weight and insulin content
No significant changes in pancreatic weight were observed between lean mice and high-fat-fed mice, receiving either saline or [I10W]tigerinin-1R injections (Fig. 4c). A significant increase (P < 0.001) in pancreatic insulin content of mice fed a high-fat diet was observed compared to lean mice (64 % increase in pancreatic insulin content). Treatment with [I10W]tigerinin-1R decreased the pancreatic insulin content in high-fat-fed mice (16 % decrease compared with high-fat-fed controls, P < 0.05) (Fig. 4d).
Effects of [I10W]tigerinin-1R on indirect calorimetry, energy expenditure and body composition
Administration of [I10W]tigerinin-1R did not have any significant effects on oxygen consumption or carbon dioxide production (data not shown). Respiratory exchange ratio (RER) of animals in high-fat diet was lower compared to that of animals fed on normal rodent diet, but administration of [I10W]tigerinin-1R had no significant effect (data not shown). Similarly, no significant changes in energy expenditure were observed during the 22-h observation period in mice treated with the peptide compared with the high-fat-fed control.
Body fat composition was significantly (P < 0.001) greater in high-fat-fed mice compared to mice on normal rodent diet (Fig. 5a, c, d). A pronounced reduction in body fat (P < 0.05) was observed in mice treated with [I10W]tigerinin-1R compared to high-fat-fed control (20 % decrease in total body fat compared to high-fat-fed control, P < 0.05, Fig. 5d). No significant differences were observed in lean body mass, bone mineral density, bone mineral content or bone area between saline- and peptide-treated group (Fig. 5B, e–g).
Effects of [I10W]tigerinin-1R on body composition in high-fat-fed mice. Mice were treated with twice-daily injections of saline or [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days prior to DEXA scan (a) and computation of data on lean body mass (b), body fat (c), body fat as % of total body mass (d), bone mineral density (e), bone mineral content (f) and bone area (g). Values are mean ± SEM for 8 mice. *P < 0.05 and ***P < 0.001 compared to lean control. Δ P < 0.05 compared to high-fat-fed control
Effects of [I10W]tigerinin-1R on the plasma lipid profile in high-fat-fed mice
High-fat-fed mice treated with saline showed significant (P < 0.01) increase in plasma triglyceride concentrations compared with mice fed a normal rodent diet (Fig. 6a). Treatment with [I10W]tigerinin-1R normalised plasma triglyceride concentrations. High-fat-fed mice also showed significant (P < 0.05) increases in total cholesterol concentrations and low-density lipoproteins (LDL) compared with lean mice. Treatment with [I10W]tigerinin-1R had no significant effects on these parameters (Fig. 6b, d). Concentrations of high-density lipoproteins (HDL) were significantly (P < 0.05) lower in high-fat-fed mice compared with lean controls, with no effect of peptide treatment (Fig. 6c).
Effects of [I10W]tigerinin-1R on plasma triglycerides (a), total cholesterol (b), HDL cholesterol (c) and LDL cholesterol (d) in high-fat-fed mice. Mice were treated with twice-daily injections of saline or [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days prior to measurement of the triglyceride and cholesterol concentrations. Values are mean ± SEM for 8 mice. *P < 0.05 and **P < 0.01 compared to normal controls
Effects of [I10W]tigerinin-1R on islet morphology, beta cell and alpha cell area
The number of islets per mm2 of pancreas was similar in lean and high-fat-fed mice (Fig. 7e). A significant increase in islet area was observed in mice fed a high-fat diet compared with normal controls (126 % increase in islet area, P < 0.001, Fig. 7b). Significant (P < 0.001) increases in beta cell and alpha cell areas were also observed in islets of high-fat-fed mice compared to normal controls (Fig. 7c, d). Treatment with [I10W]tigerinin-1R produced a significant decrease (23 %, P < 0.005) in islet area of the high-fat-fed mice. This reduction arose from decreases in both beta cell and alpha cell areas (P < 0.05).
Effects of [I10W]tigerinin-1R treatment on islet morphology (a) and computed islet area (b), alpha cell area (c), beta cell area (d) and number of islets per mm2 of pancreas (e) in high-fat-fed mice. Mice were treated with twice-daily injections of saline or [I10W]tigerinin-1R (75 nmol/kg body weight) for 28 days prior to excision of pancreata for immunohistological analysis. Values are mean ± SEM for 8 observations (~120 islets per group). **P < 0.01 and ***P < 0.001 compared to normal saline control. Δ P < 0.05 compared to high-fat-fed control
Discussion
NIH Swiss mice fed a high-fat diet represent a valuable model of diet-induced obesity and diabetes [25]. Several studies investigating the acute effects of various amphibian peptides in high-fat-fed mice, including alyteserin-2a [26] and tigerinin-1R [13], have been reported. [I10W]tigerinin-1R has been previously shown to possess increased insulinotropic potency in vitro compared with the native peptide [17]. The present study extends this work by examining the in vivo effects of twice-daily administration of [I10W]tigerinin-1R (75 nmol/kg body weight) to high-fat-fed mice over 28 days. Consistent with previous reports [27, 28], food intake, body weight, plasma glucose and insulin concentrations were all significantly greater in mice fed a high-fat diet compared with mice fed a normal rodent diet. Compared with the previous observation for the native tigerinin-1R, increase in non-fasting plasma insulin in mice treated with [I10W]tigerinin-1R is of a greater magnitude [14].
Several amphibian skin peptides have been shown to have beneficial effects on satiety and feeding activity. For example, bombesin and caerulein isolated from skin of Bombina orientalis and Litoria caerulea, respectively, have been shown to suppress food intake [29, 30]. In the present study, treatment of high-fat-fed mice with twice-daily injections of [I10W]tigerinin-1R did not result in any significant changes in energy intake compared to high-fat-fed controls. Total body weight, lean body mass and bone indices were also unchanged by [I10W]tigerinin-1R but, in contrast to the findings with administration of native tigerinin-1R [14], body fat was significantly decreased which might be expected to contribute to improvements in glucose homoeostasis.
A significant enhancement of intraperitoneal and oral glucose tolerance was observed in high-fat-fed mice treated with [I10W]tigerinin-1R. Consistent with previous observations with tigerinin-1R administration [14], this was accompanied by a significant increase in non-fasting plasma insulin and decrease in plasma glucose concentrations in peptide-treated mice from approximately day 12 onwards. In addition to confirming the acute effects of the peptide on basal glucose in vivo, these results suggest that the peptide also lowers post prandial glucose levels, an effect that has been previously reported for GLP-1 [31].
One of the major characteristics of T2DM is insulin resistance in which the sensitivity of peripheral tissues to insulin and the resultant cellular glucose uptake are impaired [32]. Acute administration of insulin (25 IU/kg body weight) induced a significantly greater decrease in plasma glucose concentration in animals treated with [I10W]tigerinin-1R compared with high-fat-fed controls, indicative of enhanced insulin sensitivity. This improvement, associated with freedom from high-fat-induced downregulation of insulin receptor (Insr) or GLUT4 (Slc2a4) in skeletal muscle, was confirmed by HOMA IR analysis. This contrasts with the results of long-term treatment with native tigerinin-1R which failed to enhance insulin action [14]. The other major hallmark of T2DM is impaired insulin secretion by pancreatic beta cells [32], as exemplified in this study by the poor insulin secreting responses of islets isolated from high-fat-fed mice. However, treatment with [I10W]tigerinin-1R, as in the previous study using tigerinin-1R [14], significantly enhanced the effects of established secretagogues, indicating that the amphibian peptide exerted beneficial actions on the dysfunctional islets isolated from mice challenged with high-fat diet.
In addition to insulin resistance, increased glucagon production observed in T2DM serves to increase glucose levels observed in T2DM by stimulating hepatic glucose output [33]. As expected, glucagon levels were elevated in mice fed a high-fat diet but treatment with [I10W]tigerinin-1R restored both circulating and pancreatic glucagon to normal levels. Pancreatic weights were similar in all groups of mice but insulin content was significantly greater in high-fat-fed animals, reflecting increased insulin demand due to insulin resistance. Consistent with this interpretation, pancreatic insulin content of high-fat-fed mice was significantly decreased by treatment with [I10W]tigerinin-1R, correlating with improved insulin sensitivity. This response also contrasts with the effects of tigerinin-1R treatment which increased insulin content in the absence of a beneficial effect on insulin action [14].
Evaluation of oxygen consumption (VO2) and carbon dioxide production (VCO2) revealed no significant differences in high-fat-fed mice treated with [I10W]tigerinin-1R compared to controls. However, RER was decreased in high-fat-fed animals as a result of increased fat metabolism as described elsewhere [34]. Consistent with the lack of effects of [I10W]tigerinin-1R on body weight and food intake, the peptide did not influence fuel selection as judged by RER.
Prolonged consumption of a diet rich in fat has been shown to induce adipose tissue deposition and increase cellular levels of triglycerides [19, 35]. In the present study, total body fat content was significantly increased in high-fat-fed mice compared to animals fed normal rodent diet. Treatment with [I10W]tigerinin-1R significantly reduced the body fat content without overall effect on total body weight. No significant change in body fat was observed in mice treated with the native tigerinin-1R under similar experimental conditions. Reduced adipose tissue deposition may also contribute to improved insulin sensitivity and insulin secretory responses observed in [I10W]tigerinin-1R-treated mice. High-fat-fed mice also exhibited increased concentrations of circulating triglycerides and cholesterol and decreased levels of HDL cholesterol. The increase in triglycerides was alleviated by treatment with [I10W]tigerinin-1R. However, treatment with the peptide did not have any effect on circulating total, HDL or LDL cholesterol concentrations.
Mice fed a high-fat diet partly compensate for the induced insulin resistance through positive changes in islet morphology [36]. In the present study, we observed an increase in islet area, and both beta cell and alpha cell areas in high-fat-fed mice compared to normal controls, correlating with changes in pancreatic hormonal contents. Consistent with improved insulin sensitivity and reduced beta cell demand, [I10W]tigerinin-1R treatment resulted in significant decreases of islet area, beta cell area and alpha cell area together with decreased pancreatic insulin and glucagon stores. Similar observations have been made in animals treated with GIP antagonists, which act to alleviate insulin sensitivity [37]. Consistent with this, no significant change in beta cell number was observed following culture of BRIN-BD11 cells with [I10W]tigerinin-1R, suggesting that the decrease in islet area [I10W]tigerinin-1R-treated mice is unlikely to reflect direct effects negative of the peptide on the islet cell growth and viability. Further, no significant differences in islet number were observed in the pancreatic tissues of normal or high-fat-fed mice.
In conclusion, the present study has demonstrated significant beneficial metabolic effects of [I10W]tigerinin-1R in mice with diet-induced obesity and insulin resistance. In addition to improving glucose tolerance and glucose-stimulated insulin secretion, the peptide enhances pancreatic beta cell function as well as insulin sensitivity and has beneficial effects on islet morphology and lipid profile. The improved efficacy of [I10W]tigerinin-1R observed in this study is believed to be as a result of its ability to improve insulin sensitivity and promote weight loss while exhibiting a superior insulinotropic effect compared to the native peptide [14]. These effects encourage further investigations of the antidiabetic potential of [I10W]tigerinin-1R, comparison against existing peptide therapies and the mechanisms underlying the improvements in both insulin secretion and action.
References
Amin RH, Chen H, Veluthakal R, Silver RB, Li J, Li G, Kowluru A (2003) Mastoparan-induced insulin secretion from insulin-secreting βTC3 and INS-1 cells: evidence for its regulation by Rho subfamily of G proteins. Endocrinology 144:4508–4518
Mohamed AH, El-Serougi M, Kamel A (1965) Effect of Walterinnesia aegypta venom on blood glucose in diabetic animals. Toxicon 3:163–165
Varga Z, Gurrola-Briones G, Papp F, Rodriguez de la Vega RC, Pedeaza-Alva G, Tajhya RB, Gaspar R, Cardenas L, Rosenstein Y, Beeton C, Possani LD, Panyi G (2012) Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol Pharmacol 82:372–382
Roudbari L, Imani S (2012) The effects of Androctonus crassicauda scorpion venom in the treatment of diabetes mellitus type 1 in animal models. Ann Biol Res 3:5782–5785
Egan J, Clocquet A, Elahi D (2002) The insulinotropic effect of acute exendin-4 administered to humans: comparison of non-diabetic state to type 2 diabetes. J Clin Endocrinol Metab 87:1282–1290
Parkes DG, Mace KF, Trautmann ME (2013) Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov 8:219–244
Hansen KB, Knop FK, Holst JJ, Vilsboll T (2009) Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int J Clin Pract 63:1154–1160
Conlon JM, Mechkarska M, Luki ML, Flatt PR (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 57:67–77
Sai KP, Jagannadhan MV, Vairamani M, Raju NP, Devi AS, Nagaraj R, Sitaram N (2001) Tigerinins: novel antimicrobial peptides from the Indian frog Rana tigerina. J Biol Chem 276:2701–2707
Song Y, Lu Y, Wang L, Yang H, Zhang K, Lai R (2009) Purification, characterization and cloning of two novel tigerinin-like peptides from skin secretions of Fejervarya cancrivora. Peptides 30:1228–1232
Conlon JM, Raza H, Coquet L, Jouenne T, Leprince J, Vaudry H, King JD (2009) Purification of peptides with differential cytolytic activities from the skin secretions of the Central American frog, Lithobates vaillanti (Ranidae). Comp Biochem Physiol C Toxicol Pharmacol 150:150–154
Mechkarska M, Ahmed E, Coquet L, Leprince J, Jouenne T, Vaudry H, King JD, Conlon JM (2011) Peptidomic analysis of skin secretions demonstrates that the allopatric populations of Xenopus muelleri (Pipidae) are not conspecific. Peptides 32:1502–1508
Ojo OO, Abdel-Wahab YHA, Flatt PR, Mechkarska M, Conlon JM (2011) Tigerinin-1R: a potent, non-toxic insulin-releasing peptide isolated from the skin of the Asian frog, Hoplobatrachus rugulosus. Diabetes Obes Metab 13:1114–1122
Ojo OO, Srinivasan DK, Owolabi BO, Flatt PR, Abdel-Wahab YHA (2015) Beneficial effects of tigerinin-1R on glucose homeostasis and beta cell function in mice with diet-induced obesity-diabetes. Biochimie 109:18–26
Ojo OO, Conlon JM, Flatt PR, Abdel-Wahab YHA (2013) Frog skin peptides (tigerinin-1R, magainin-AM1, CPF-AM1 and PGLa-AM1) stimulate secretion of glucagon-like peptide 1 (GLP-1) by GLUTag cells. Biochem Biophys Res Commun 431:14–18
Pantic JM, Mechkarska M, Lukic ML, Conlon JM (2014) Effects of tigerinin peptides on cytokine production by mouse peritoneal macrophages and spleen cells and by human peripheral blood mononuclear cells. Biochimie 101:83–92
Srinivasan D, Ojo OO, Abdel-Wahab YHA, Flatt PR, Guilhaudis L, Conlon JM (2014) Insulin-releasing and cytotoxic properties of the frog skin peptide, tigerinin-1R: a structure-activity study. Peptides 55:23–31
Martin CMA, Irwin N, Flatt PR, Gault VA (2013) A novel acylated form of (D-Ala2) GIP with improved antidiabetic potential, lacking effect on body fat stores. Biochim Biophys Acta 1830:3407–3413
Gault VA, Porter DW, Irwin N, Flatt PR (2011) Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1–30) and GIP(1–42) in high-fat fed mice. J Endocrinol 208:265–271
Vasu S, Moffett RC, McClenaghan NH, Flatt PR (2015) Responses of GLP1-secreting L-cells to cytotoxicity resemble pancreatic β-cells but not α-cells. J Mol Endocrinol 54:91–104
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact Islets of Langerhans from the rat pancreas. Diabetes 16:35–39
Flatt PR, Bailey CJ (1981) Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20:573–577
Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM (2014) Activation of GPR119 by fatty acid agonists augments insulin release from clonal B-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol Chem 395:453–464
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53:S215–S219
Ojo OO, Abdel-Wahab YHA, Flatt PR, Conlon JM (2013) Insulinotropic actions of the frog skin host-defense peptide alyteserin-2a: a structure—activity study. Chem Biol Drug Des 82:196–204
Martin CMA, Gault VA, McClean S, Flatt PR, Irwin N (2012) Degradation, insulin secretion, glucose-lowering and GIP additive actions of palmitate-derivatised analogues of xenin-25. Biochem Pharmacol 84:312–319
Porter D, Faivre E, Flatt PR, Holscher C, Gault VA (2012) Actions of incretin metabolites on locomotor activity, cognitive function and in vivo hippocampal synaptic plasticity in high fat fed mice. Peptides 35:1–8
Griesbacher T, Leighton GE, Hill RG, Hughes J (1989) Reduction of food intake by central administration of cholecystokinin octapeptide in the rat is dependent upon inhibition of brain peptidases. Br J Pharmacol 96:236–242
Ladenheim EE, Hampton LL, Whitney AC, White WO, Battey JF, Moran TH (2002) Disruptions in feeding and body weight control in gastrin-releasing peptide receptor deficient mice. J Endocrinol 174:273–281
Fineman MS, Cirincione BB, Maggs D, Diamant M (2012) GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab 14:675–688
Leahy JL (2005) Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209
D’Alessio D (2011) The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab 13:126–132
Church C, Lee S, Bagg EAL, McTaggart JS, Deacon R, Gerken T, Lee A, Moir L, Mecinovic J, Quwailid MM, Schofield CJ, Ashcroft FM, Cox RD (2009) A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 5:e1000599. doi:10.1371/journal.pgen.1000599
McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR (2007) GIP receptor antagonism reverses obesity, insulin resistance and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 293:1746–1755
Zhang J, Chen L, Zheng J, Zeng T, Li H, Xiao H, Deng X, Xu X (2012) The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res Clin Pract 97:474–482
Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, Harriott P, O’Harte FP, Flatt PR (2005) Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorated insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 54:2436–2446
Acknowledgments
Funding for this study was provided by a project grant from Diabetes UK (Grant Number 12/0004457) and an award of a University Vice Chancellor Research Studentship to DKS.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63EU for animal experiments. In addition, all applicable institutional guidelines for the care and use of experimental animals were followed.
Human and animal rights disclosure
This article does not contain any studies with human subjects performed by the any of the authors.
Informed consent
No informed consent is required.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Srinivasan, D.K., Ojo, O.O., Owolabi, B.O. et al. [I10W]tigerinin-1R enhances both insulin sensitivity and pancreatic beta cell function and decreases adiposity and plasma triglycerides in high-fat mice. Acta Diabetol 53, 303–315 (2016). https://doi.org/10.1007/s00592-015-0783-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0783-3