Abstract
Introduction
Chronic osteomyelitis (COM) is a devastating infection requiring a multidisciplinary approach, including radiology, microbiology, pathology, and orthopaedic surgery to treat. The present study analysed the bacterial profile causing chronic osteomyelitis and their antibiogram in our region.
Patients and methods
This prospective study was done on a consecutive group of patients who underwent surgical debridement for long bone COM. Three to six deep tissue samples were collected during the index debridement for microbiology and one sample for histopathology. Antimicrobial sensitivity testing used an automated bacterial identification system. Gram stain was used to identify the bacteria type from its size, shape, and arrangement of bacterial growth.
Results
Intra-operative deep tissue and bone specimens accurately identified causative bacteria in 84.8% of patients. Gram-ve bacilli (GNB) were the most common causative organisms in 51.6% of all growing samples (36.4% isolated G-ve and 15.2% mixed with G + ve). Thirty-three patients (30 males/three females) were included; the mean age at index debridement surgery was 37.1 years. Half of the cohort had no metalwork. The aetiology of COM was post-operative infection in half of the patients.
Conclusion
There may be concerning features in our patients’ aetiologies and causative organisms; closed fractures turn into COM postoperatively, several unsuccessful attempts, delayed index debridement, and more GNB. Plans need to be applied to break the cycle and improve outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteomyelitis is characterized by low-grade inflammation caused by persistent pathogenic microorganisms with bone destruction and necrosis. It is still one of the most challenging conditions due to its long course, complex treatment, and risk of recurrence [1, 2]. Chronic osteomyelitis is intensely challenging to treat due to the poor blood supply, the devitalized tissues, the poor antibiotic penetration and the soft tissue envelope in some regions [1]. Biofilm-producing organisms adhere resiliently to the surface of the bone and implanted material, which makes them resistant to host defences and antibiotics [2, 3].
A definitive diagnosis of chronic osteomyelitis depends on identifying causative organisms through microbiological methods (culture and sensitivity) and histopathological examination of tissues [1]. Gram-positive organisms (specifically Staph aureus) remained the most commonly reported organisms to cause chronic osteomyelitis in long bones. However, other reports revealed paradoxical results with prevailing Gram-negative bacilli (GNB). Gram-negative osteomyelitis is hard to control and has increased multidrug resistance (MDR) and increased rates of recurrence [4,5,6,7,8,9].
This study aimed to analyse and discuss the microbiological results of intraoperatively collected samples of patients diagnosed with chronic osteomyelitis in one centre. With very few available published data from low resources regions [10], we planned to investigate the current situation to figure out the best way to control this debilitating disease and update our infection control measures.
Patients and methods
Study design
This prospective clinical study was done on a consecutive group of 33 patients who underwent surgical debridement for long bones chronic osteomyelitis in a single centre (Benha University Hospitals). The patients had their initial diagnosis and previous surgeries elsewhere in the region (Qalyubia governorate) and were referred to our centre later due to persistent infection. All patients had an established diagnosis of COM (clinical and radiological) in the appendicular system. Acute osteomyelitis or septic arthritis, periprosthetic infection, diabetic foot infection, or vertebral osteomyelitis were excluded from the study.
Preoperative assessment
All patients included in the study were evaluated clinically, laboratory, and radiologically. Laboratory investigation included a complete blood picture, erythrocyte sedimentation rate, C-reactive protein, and other relevant preoperative investigations. A detailed history of previous surgeries and the metalwork used was also obtained.
Radiologically, anteroposterior and lateral plain X-rays were obtained to evaluate bone quality, sequestrated bone, sclerosis, the union of fractures, and loosening around metalwork. 18F-Fluorodeoxyglucose (FDG)-positron emission tomography (FDG PET-CT) scan was done on 70% of patients, aiming to provide a three-dimensional analysis of the infected area, helping the preoperative planning and determine the extent of resection during surgical debridement.
Surgical debridement and samples collection
Surgical index debridement was done for all cases by the first author (AE) between February 2019 and February 2022. The extent of debridement was planned preoperatively. Antibiotic therapy was stopped 14 days before the index debridement surgery, and no routine antibiotic prophylaxis was given until bone/tissue biopsies were collected. Three to six deep bone/tissue samples were collected in leak-proof sterile containers using different instruments and immediately transferred to the Microbiology department [11].
Microbiological examination
Standard bacteriological techniques were applied to isolate and identify the causative organisms. Specimens were cultured on primary culture media (nutrient, blood, and MacConkey agars), incubated at 37 °C for 24–48 h. Liquid samples were inoculated in blood culture broths incubated in Bact/alert system. Positive vials were cultured on the previous culture media. Colonial growth on the agar surface was inspected for colonial morphology, haemolysis on blood agar, swarming, or any other characteristic odour or pigment. Where mixed bacterial growth was observed, subculture was done. No molecular typing or PCR was performed.
Antimicrobial sensitivity testing used an automated bacterial identification system (Vitek 2 and Phoenix) that analyses MIC patterns and detects phenotypes for most tested organisms. Smear from the bacterial growth was prepared and fixed with one or two drops of methanol, stained with Gram stain and examined microscopically.
Histopathological examination
One bone/soft tissue sample was sent for histopathological examination; the sample was collected and transferred immediately with no preservative fluid. The histopathologists were not aware of the microbiological results of the patients. The characteristic histopathological findings of COM are the persistence of dilated blood vessels, granulation tissue, inflammatory cell infiltration, devitalized bone, reactive new bone formation, and suppuration. A definitive diagnosis of COM was made based on deep tissue surgical cultures and histopathological analysis.
Analysis of the results and statistical methods
Data management and statistical analysis were done using SPSS version 28 (IBM, Armonk, New York, USA). Quantitative data were assessed for normality using the Shapiro–Wilk test and direct data visualization methods. According to normality testing, numerical data were summarized as means and standard deviations or medians and ranges. Categorical data were summarized as numbers and percentages. The microbiology, histopathology, and culture results were plotted together to conclude and investigate the causative organisms and antibiotic culture and sensitivity.
Results
This study was conducted on 33 patients (30 males and three females) suffering from chronic osteomyelitis (COM). There were 18 smokers, one EX smoker, and 14 nonsmokers. The mean age at the index debridement surgery was 37.1 years (range 7–73). Patient characteristics are detailed in Table 1.
Laboratory and culture findings
The mean WBCs were 7.1 ± 1.1. The mean ESR was 48.8 ± 12.2. The mean CRP was 22.3 ± 9.7. The mean number of samples collected for microbiology was 5 ± 1. Growth was reported in 28 patients (84.8%). GNB were the most common causative organisms in 51.5% of the cohort, slightly higher than Gram-positive (48.5%). Looking at the spectrum of organisms, cultures grew isolated GNB in 36.4%, gram-positive in 33.3%, mixed in 15.2%, and no growth in 15.2%. Monomicrobial infection was found in 18 patients (54.5%), while polymicrobial infection (different identified organisms) was evident in a third of the cohort (ten patients, 30.3%) (Table 2).
Organism identity and sensitivity
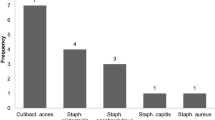
MRSA was the most frequently identified single organism to grow in all cultures (isolated or mixed) (36.4%). Klebsiella was the most frequent single GNB (15.2%); However, there were some results of Gram-negative cultures (12.1%) that could not identify the organism specifically (Table 3). MDR Klebsiella (n = 5) was only 20–40% sensitive to gentamycin, amikacin, ciprofloxacin, and trimethoprim–sulphamethoxazole. Pseudomonas (n = 3) was 100% sensitive towards piperacillin/tazobactam, gentamycin, amikacin, ciprofloxacin, and levofloxacin and 66% sensitive to cefipime and imipenem. E. Coli (n = 3) was sensitive to piperacillin–tazobactam, ciprofloxacin, and levofloxacin in 66.7% and sensitive to amoxicillin–clavulanic acid and trimethoprim–sulphamethoxazole in only 33.3%. Proteus (n = 3) was 67.3% sensitive to piperacillin/tazobactam, meropenem, ceftazidime, and amikacin.
Discussion
In this study, intra-operative deep tissue and bone specimens accurately identified causative bacteria in 84.8% of patients. All cases underwent index debridement and sample collection by the same surgeon, with three to six collected specimens. There was an increased incidence of G-negative osteomyelitis in 51.5% of the whole cohort, either as isolated GNB (36.4%) or mixed gram-positive and negative (15.2%).
Chronic osteomyelitis is a challenge for orthopaedic surgeons. Appropriate diagnosis and treatment of chronic osteomyelitis require microbiologic cultures of the infected bone and proper antibiotics according to culture and sensitivity. Bone provides a unique harbour for microorganisms that produce biofilms, allowing them to attach resiliently to biologic and implanted surfaces, remaining insusceptible to host defences and antibiotics [2].
Several studies analysed the bacteriological profile in long bone osteomyelitis [3, 5,6,7,8, 12,13,14,15,16]. Reports from low resources settings and developing countries are sparse [4, 9, 10, 17, 18]. Nevertheless, other studies investigated the aetiology and bacteriology of COM in a diabetic foot or joint replacement infection or mixed group of patients [19,20,21]. We did not reflect on these studies due to the particular pathology in each situation which is different from implant-related long bone osteomyelitis.
Sheehy et al. and Dudareva et al. reported 66% and 67.7% culture-positive results, respectively, compared to 84.4% in this study [12, 15]. Sheehy et al. reported a range of samples from 0 to 10, with no samples analysed in ten patients out of 166 (6%). Marais et al. [7]. recorded higher culture-positive results (90.9%); however, this result represents only 11 of 26 patients who underwent surgical debridement and intra-operative bone biopsies.
The lack of growth in the collected samples of any study has some explanations. Antibiotics should be stopped for 14 days in clinically stable patients before surgery. Samples should be collected from the deep bone and soft tissues. Multiple samples should be collected with different instruments. Proper containers and a timely chain of transfer should be applied. However, we applied all these precautions in this study, with 15% of samples failing to grow organisms [11].
Other factors are related to microbiological methods and techniques. Pretreatment of samples with specific chemicals could release the organism from the biofilm. Also, physical methods like sonication offer similar effects. Extended cultures also provide extra opportunities to grow weak organisms [22]. This study could not apply such methods due to unavailability or logistic problems.
Although sinus tract swabs are still one of the diagnostic tools of COM and may provide the accurate causative organism, tissue specimen remains the gold standard for diagnosing COM and providing accurate causative organisms. Vemu et al. compared tissue specimens and sinus tract swabs; positive culture in tissue specimens was 60.7% compared to 37.7% in sinus swabs [23]. Contamination with nonpathogenic bacteria or other naturally occurring random organisms during the sampling process could explain this outcome.
The current study showed that chronic osteomyelitis was the highest in middle age (median 37y), which agrees with other studies [4, 7, 15, 23]. Road traffic accidents frequently occur among those age groups; such high-energy trauma often leads to open fractures and soft tissue trauma and potential risk factors for traumatic osteomyelitis. In the current study, the male/female ratio was 10:1, which is very similar to previous studies [7, 12, 13, 15, 23].
The aetiology of COM in our study was post-operative in half of the patients. In other studies, haematogenous osteomyelitis or OM following open fractures were the leading cause [13, 15, 16]. Post-operative COM was only 29.3%, 9.9%, and 2.8% of the whole cohorts, respectively [4, 12, 13]. This finding is very critical, we had difficulty analysing this high rate, but there were some clues. Patients were operated on previously in different hospitals. Also, there is no universal MRSA screen or urine dipstick screening. We could not document the general health status or the soft tissue injury. Additionally, antibiotic and follow-up regimens were different, with a tendency to give strong antibiotics for a long time and to do daily dressing on the post-operative wounds. Finally, we do not have the number of surgeries at each hospital to get an actual incidence of surgical site infection.
In the current study, the tibia (48.5%) was the most frequent site of chronic osteomyelitis. Many studies had similar findings [4, 7, 12, 13, 15, 16]. Two other studies reported the femur as the most frequent infected bone [14, 23]; in our report, the femur was the second affected bone (30.3%).
GNB organisms were the most common cultured bacteria in 51.5% of patients (alone or mixed with Gram + ve organisms). In the literature, GNB bacteria were prevalent in a few studies. GNB incidence has evolved; a historical paper by Meyers et al. reported a 28% incidence of GNB in 1973, increasing from 15% only in 1966 [8]. Recently, Marais et al. reported 26 patients in South Africa with COM in two groups: palliative versus curative. Their cohort had nearly equal numbers of haematogenous, post-operative and open fractures, and the tibia was the most commonly affected bone. GNB infection was recorded in 61.5% (n = 8) compared to 38.5% (n = 5) Gram + ve [7].
In Egypt, two studies analysed the bacteriological profile in their centres within the context of reporting the surgical outcomes of single-stage debridement [17, 18]. El-Moatassem examined 16 patients with tibial COM, who grew Gram + ve bacteria (MSSA and MRSA) in 12 (75%) and Pseudomonas in one; three more did not grow any organism [18]. Badie and Arafa reported the results of 30 cases with long bone COM; half had tibial involvement. They had 56% haematogenous, 20% post-operative infection, and the rest were open fractures. Cultures were positive in 93% of cases, and GNB grew in 32% [17]. Both studies emphasized the surgical aspects; we could not retrieve further details on sensitivity or resistance patterns.
Agaja and Ayorinde reported similar trends in their cohort of 107 patients from Nigeria, with GNB (43%) slightly higher than Gram + ve (37.4%). However, 81.3% of their patients had haematogenous osteomyelitis [4]. Mousa et al. studied 134 patients from Iraq in four groups: haematogenous, exogenous (open fractures), post-operative, and mastoiditis. GNB (Pseudomonas species and Klebsiella species) were prevalent in the exogenous and post-operative osteomyelitis groups [9].
Carvalho et al. published a study that included 101 patients from Brazil who presented with isolated GNB osteomyelitis; 43% COM and 32% were associated with open fractures, and the remaining were post-operative. Clinical remission was achieved in 60% of patients. Enterobacter and Acinetobacter baumannii were the commonest. Pseudomonas came third, with sensitivity ranging from 70 to 80% towards the GNB targeting antibiotics [5]. Jorge et al. investigated 193 patients diagnosed with osteomyelitis following fractures (closed or open) in Brazil. They reported GNB in 51.8% of patients. Pseudomonas aeruginosa and A. baumannii were the most frequent [14].
Koutserimpas et al. analysed 14 patients with MDR and extended drug resistance (XDR) in one centre in Greece over ten years; most had post-operative osteomyelitis. Acinetobacter baumannii was the most frequent, followed by Escherichia coli and Klebsiella pneumoniae. They highlighted that A. baumanii was repeatedly a leading cause of hospital-acquired infection in their region [6]. In our cohort, Klebsiella was the commonest, followed by Pseudomonas, Proteus and Ecoli. Acinetobacter was found in one patient with no Enterobacter.
In the present study, MRSA remains the most frequent single isolate at 36.4% (n = 12), in agreement with many previous studies, which reported an incidence ranging between 20 and 48% [4, 12, 13, 15, 16, 23]. However, two studies from the same team ten years apart [12, 15] reported very similar incidences of staphylococcus infections (31.3%–37.5%) in both cohorts, yet, there was a declining proportion of MRSA from 30.8 to 11.4% of all staph infections, respectively. Strict applied hospital infection prevention policies could have resulted in this reduction, including preoperative MRSA screening and decolonization with topical nasal mupirocin and chlorhexidine shower.
In the current study, 85.7% of S. aureus strains were resistant to methicillin (MRSA). In addition, all MRSA were 100% sensitive to amikacin, ciprofloxacin, erythromycin, linezolid, vancomycin, and clindamycin. This spectrum of sensitivities draws attention to the current problem and should attract more practical steps to reduce MRSA osteomyelitis. GNB sensitivity profile also represents a challenge; GNB is more likely to be resistant to multiple drugs, e.g. Klebsiella, which is 40% sensitive only to gentamicin and amikacin and 20% sensitive to ciprofloxacin and sulphamethoxazole.
In this study, we aimed to raise voices about the differences in causes and bacteriological profiles of COM in low resources settings. The management of chronic osteomyelitis in low resources countries had more obstacles compared to the developed world [10]. However, the high-quality literature about the bacteriological spectrum and sensitivities profile was driven by developed countries [12, 15]. Consensus and recommendations for antibiotic management were also reported from specialized centres in high-income countries [24, 25]. The contradictory findings in this study would change the current practice of choice of antibiotic prophylaxis and treatment in low resources settings.
Limitations
This study has some limitations due to the relatively small sample size, heterogeneity of cases and single hospital location. The lack of data about the previous surgeries in different hospitals made tracing challenging. We could not identify the causes behind the increased post-operative infection which is a major preventable source in our cohort. More work needs to be done to analyse the GNB endemic and how to fight it.
Conclusion
There may be a shift in our region’s aetiologies and causative organisms; closed fractures turn into COM postoperatively, several unsuccessful attempts, delayed index debridement, and more GNB organisms. Plans need to be applied to break the cycle and improve outcomes.
References
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364(9431):369–379. https://doi.org/10.1016/S0140-6736(04)16727-5
Masters EA, Trombetta RP, de Mesy Bentley KL, Boyce BF, Gill AL, Gill SR et al (2019) Evolving concepts in bone infection : redefining “biofilm”,“acute versus chronic osteomyelitis”,“the immune proteome” and “local antibiotic therapy.” Bone research 7(1):1–18. https://doi.org/10.1038/s41413-019-0061-z
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(9276):135–138. https://doi.org/10.1016/s0140-6736(01)05321-1
Agaja SB, Ayorinde RO (2008) Chronic osteomyelitis in Ilorin, Nigeria. South Afr J Surg Suid-Afrik Tydskr Chir 46(4):116–118
Carvalho VC, Oliveira PR, Dal-Paz K, Paula AP, Felix Cda S, Lima AL (2012) Gram-negative osteomyelitis: clinical and microbiological profile. Braz J Infect Dis : Off Publ Braz Soc Infect Dis 16(1):63–67
Koutserimpas C, Samonis G, Plataki MN, Bikis C, Kontakis G, Kofteridis DP (2018) Multidrug-resistant Gram-negative osteomyelitis: a 10-year study. Il G Chir 34(5):284–290
Marais LC, Ferreira N, Aldous C, Le Roux TL (2016) The outcome of treatment of chronic osteomyelitis according to an integrated approach. Strateg Trauma Limb Reconstr 11(2):135–142. https://doi.org/10.1007/s11751-016-0259-1
Meyers BR, Berson BL, Gilbert M, Hirschman SZ (1973) Clinical patterns of osteomyelitis due to gram-negative bacteria. Arch Intern Med 131(2):228–233
Mousa HA, Hamdan TA, Bakr SS (2001) Clinical and microbiological evaluation of osteomyelitis. Bahrain Med Bull 23(2):61–65
Tissingh EK, Marais L, Loro A, Bose D, Paner NT, Ferguson J et al (2022) Management of fracture-related infection in low resource settings: how applicable are the current consensus guidelines? EFORT Open Rev 7(6):422–432. https://doi.org/10.1530/eor-22-0031
Sousa R, Carvalho A, Santos AC, Abreu MA (2021) Optimal microbiological sampling for the diagnosis of osteoarticular infection. EFORT Open Rev 6(6):390–398. https://doi.org/10.1302/2058-5241.6.210011
Dudareva M, Hotchen AJ, Ferguson J, Hodgson S, Scarborough M, Atkins BL et al (2019) The microbiology of chronic osteomyelitis: changes over 10 years. J Infect 79(3):189–198. https://doi.org/10.1016/J.JINF.2019.07.006
Garcia Del Pozo E, Collazos J, Carton JA, Camporro D, Asensi V (2018) Factors predictive of relapse in adult bacterial osteomyelitis of long bones. BMC Infect Dis 18(1):635. https://doi.org/10.1186/s12879-018-3550-6
Jorge LS, Fucuta PS, Oliveira MGL, Nakazone MA, de Matos JA, Chueire AG et al (2018) Outcomes and risk factors for polymicrobial posttraumatic osteomyelitis. J Bone Jt Infect 3(1):20–26. https://doi.org/10.7150/jbji.22566
Sheehy SH, Atkins BA, Bejon P, Byren I, Wyllie D, Athanasou NA et al (2010) The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 60(5):338–343. https://doi.org/10.1016/j.jinf.2010.03.006
Zhang Z, Liu P, Wang W, Wang S, Li B, Li J et al (2022) Epidemiology and drug resistance of fracture-related infection of the long bones of the extremities: a retrospective study at the largest trauma center in Southwest China. Front Microbiol 13:923735. https://doi.org/10.3389/fmicb.2022.923735
Badie AA, Arafa MS (2019) One-stage surgery for adult chronic osteomyelitis: concomitant use of antibiotic-loaded calcium sulphate and bone marrow aspirate. Int Orthop 43(5):1061–1070. https://doi.org/10.1007/s00264-018-4063-z
El-Moatassem E-H (2012) A single-stage operation in the treatment of tibial chronic osteomyelitis with the use of the Ilizarov technique. Egypt Orthop J 47(3):296–304. https://doi.org/10.7123/01.eoj.0000417995.30471.20
Jiang N, Ma YF, Jiang Y, Zhao XQ, Xie GP, Hu YJ et al (2015) Clinical characteristics and treatment of extremity chronic osteomyelitis in Southern China: a retrospective analysis of 394 consecutive patients. Medicine 94(42):e1874. https://doi.org/10.1097/MD.0000000000001874
Ma X, Han S, Ma J, Chen X, Bai W, Yan W et al (2018) Epidemiology, microbiology and therapeutic consequences of chronic osteomyelitis in northern China: a retrospective analysis of 255 patients. Sci Rep 8(1):14895. https://doi.org/10.1038/s41598-018-33106-6
Schindler M, Gamulin A, Belaieff W, Francescato M, Bonvin A, Graf V et al (2013) No need for broad-spectrum empirical antibiotic coverage after surgical drainage of orthopaedic implant infections. Int Orthop 37(10):2025–2030. https://doi.org/10.1007/s00264-013-1924-3
Drago L, Clerici P, Morelli I, Ashok J, Benzakour T, Bozhkova S et al (2019) The World Association against infection in orthopaedics and trauma (WAIOT) procedures for microbiological sampling and processing for periprosthetic joint infections (PJIs) and other implant-related infections. J Clin Med. https://doi.org/10.3390/jcm8070933
Vemu L, Sudhaharan S, Mamidi N, Chavali P (2018) Need for appropriate specimen for microbiology diagnosis of chronic osteomyelitis. J Lab Physicians 10(1):21–25. https://doi.org/10.4103/JLP.JLP_14_17
Baertl S, Walter N, Engelstaedter U, Ehrenschwender M, Hitzenbichler F, Alt V et al (2022) What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics (Basel, Switzerland). 11(3). https://doi.org/10.3390/antibiotics11030287
Li H-K, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, et al. (2019) Oral versus Intravenous Antibiotics for Bone and Joint Infection. New England J Med 380(5):425–36. https://doi.org/10.1056/NEJMoa1710926
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AE conceived the original idea. All authors approved the proposal and designed the analysis. AE, AH, and MK collected the data. AE and AH performed an analysis. The first draft of the manuscript was written by AH and edited by AE. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interests.
Ethical standard
Research Ethics Committee (REC) approval no. (MS 18–7-2020), Faculty of Medicine, Benha University, Fareed Nada street, Benha, 13511, Egypt.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsheikh, A., Hashish, A., Kamal, M. et al. Aetiology of long bone chronic osteomyelitis: an analysis of the current situation in one region in Egypt. Eur J Orthop Surg Traumatol 33, 507–513 (2023). https://doi.org/10.1007/s00590-022-03429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-022-03429-2