Abstract
Background
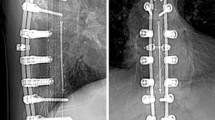
Success after glenoid bone augmentation in total shoulder arthroplasty depends on osseous integration and non-resorption. Standard imaging techniques, such as computed tomography (CT) and X-rays, cannot quantify bone viability. Therefore, we introduce a new technique to assess graft viability using 18F-sodium fluoride (18F-NaF) PET–CT for femoral allografts in reverse total shoulder arthroplasty (RSA).
Materials and methods
Patient charts were reviewed following glenoid augmentation using femoral allografts in reverse total shoulder arthroplasty. A total of seven patients were included in this study. 18F-NaF PET–CT was used to assess graft viability and graft fusion. Semiquantitative assessment of 18F-NaF uptake was performed by means of a standardized uptake value (SUV). Radiographs were used to assess fusion. The mean age of the patients at the time of follow-up was 83.4 years (range 79–92), and the mean follow-up was 44.4 months.
Results
Viability and fusion were confirmed in all allografts using semiquantitative analysis of 18F-NaF PET–CT by means of standardized uptake value (SUVmax). Metabolic activity of medullary region of a vertebral spine was defined as a reference background. The mean value of maximum tracer activity in the allograft was not statistically different from native bone in the reference vertebrae (p = 0.14).
Conclusions
18F-NaF PET–CT is a practicable tool to quantitatively assess viability in large bone allografts after glenoid augmentation in RSA. The study shows viability and fusion in all allografts.
Level of Evidence
Level IV, treatment study.




Similar content being viewed by others
References
Boileau P, Avidor C, Krishnan SG, Walch G, Kempf J-F, Molé D (2002) Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elb Surg 11:351–359. https://doi.org/10.1067/mse.2002.125807
Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH (2009) Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elb Surg 18:859–863. https://doi.org/10.1016/j.jse.2008.11.020
Nowak DD, Bahu MJ, Gardner TR, Dyrszka MD, Levine WN, Bigliani LU, Ahmad CS (2009) Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elb Surg 18:680–688. https://doi.org/10.1016/j.jse.2009.03.019
Sabesan V, Callanan M, Ho J, Iannotti JP (2013) Clinical and radiographic outcomes of total shoulder arthroplasty with bone graft for osteoarthritis with severe glenoid bone loss. J Bone Jt Surg Am 95:1290–1296. https://doi.org/10.2106/JBJS.L.00097
Walch G, Moraga C, Young A, Castellanos-Rosas J (2012) Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elb Surg 21:1526–1533. https://doi.org/10.1016/j.jse.2011.11.030
Jones RB, Wright TW, Zuckerman JD (2016) Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elb Surg 25:1425–1432. https://doi.org/10.1016/j.jse.2016.01.016
Scalise JJ, Iannotti JP (2008) Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop 466:139–145. https://doi.org/10.1007/s11999-007-0065-7
Sears BW, Johnston PS, Ramsey ML, Williams GR (2012) Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg 20:604–613. https://doi.org/10.5435/JAAOS-20-09-604
Wagner E, Houdek MT, Griffith T, Elhassan BT, Sanchez-Sotelo J, Sperling JW, Cofield RH (2015) Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 97:1653–1660. https://doi.org/10.2106/JBJS.N.00732
Bateman E, Donald SM (2012) Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elb Surg 21:925–934. https://doi.org/10.1016/j.jse.2011.07.009
Iannotti JP, Frangiamore SJ (2012) Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elb Surg 21:765–771. https://doi.org/10.1016/j.jse.2011.08.069
Blumenthal SL, Gill K (1993) Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine 18:1186–1189
Fogel GR, Toohey JS, Neidre A, Brantigan JW (2008) Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: x-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J Off J N Am Spine Soc 8:570–577. https://doi.org/10.1016/j.spinee.2007.03.013
Beheshti M, Mottaghy FM, Paycha F, Behrendt FFF, Van den Wyngaert T, Fogelman I, Strobel K, Celli M, Fanti S, Giammarite F, Krause B (2015) (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur J Nucl Med Mol Imaging 42:1767–1777. https://doi.org/10.1007/s00259-015-3138-y
Cook GJ, Fogelman I (2001) The role of positron emission tomography in skeletal disease. Semin Nucl Med 31:50–61
Pumberger M, Prasad V, Druschel C, Disch AC, Brenner W, Schaser K-D (2016) Quantitative in vivo fusion assessment by (18)F-fluoride PET/CT following en bloc spondylectomy. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 25:836–842. https://doi.org/10.1007/s00586-015-4121-9
Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ (2003) Radionuclide bone imaging: an illustrative review. Radiogr Rev Publ Radiol Soc N Am Inc 23:341–358. https://doi.org/10.1148/rg.232025103
Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, Smith GT (2010) SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med Off Publ Soc Nucl Med 51:1813–1820. https://doi.org/10.2967/jnumed.110.082263
Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, Wilensky R, Newberg AB, Basu S, Langsteger W, Alavi A (2011) Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography–a novel concept. Hell J Nucl Med 14:114–120
Enneking WF, Campanacci DA (2001) Retrieved human allografts : a clinicopathological study. J Bone Joint Surg Am 83-A:971–986
Willems WF, Kremer T, Friedrich P, Bishop AT (2014) Surgical revascularization in structural orthotopic bone allograft increases bone remodeling. Clin Orthop 472:2870–2877. https://doi.org/10.1007/s11999-014-3658-y
Acknowledgements
The authors, their immediate families and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was obtained from Gemeinsame Ethikkommission der Barmherzigen Schwestern und Barmherzigen Brüder, study number: EKS 11/17.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hochreiter, J., Mattiassich, G., Hitzl, W. et al. Quantitative in vivo assessment of bone allograft viability using 18F-fluoride PET/CT after glenoid augmentation in reverse shoulder arthroplasty: a pilot study. Eur J Orthop Surg Traumatol 29, 1399–1404 (2019). https://doi.org/10.1007/s00590-019-02463-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-019-02463-x