Abstract
Purpose
Aerobic exercise produces beneficial outcomes in patients with low back pain and partially attenuates the fibrotic changes to the multifidus in a model of intervertebral disc (IVD) degeneration. More targeted exercise might be required to fully attenuate these fibrotic alterations. This study aimed to investigate whether activation of the multifidus induced by neurostimulation could reduce fibrosis of the multifidus in a model of IVD degeneration in sheep.
Methods
IVD degeneration was induced in 18 merino sheep via a partial thickness unilateral annulus fibrosus lesion to the L1/2 and L3/4 IVDs. All sheep received an implantable neurostimulation device that provides stimulation of the L2 medial branch of the dorsal ramus. Three months after surgery, the animals were assigned to Injury or Activated groups. Activated animals received neurostimulation and the Injury group received no stimulation. Six months after surgery, the multifidus was harvested at L2 and L4. Van Gieson’s, Sirius Red and immunofluorescence staining for Collagen-I and -III and quantitative PCR was used to examine fibrosis. Muscle harvested from a previous study without IVD injury was used as a control.
Results
Neurostimulation of the multifidus attenuated IVD degeneration dependent increases in the connective tissue, including Collagen-I but not Collagen-III, compared to the Injury group at L4. No measures of the multifidus muscle at L2, which received no stimulation, differed between the Injury and Activated groups.
Conclusions
These data reveal that targeted activation of the multifidus muscle attenuates IVD degeneration dependent fibrotic alterations to the multifidus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is the foremost cause of disability and loss of work hours worldwide [1]. Treatments for LBP produce beneficial outcomes, but effects are modest [2]. Despite evidence that exercise is the most effective treatment, the mechanisms are not yet resolved and likely differ between types of exercise [3]. Exercise interventions that target back muscle structure (e.g., fibrosis, fat infiltration, and muscle fibre composition changes) and function aim to improve the quality of control of spine loading and have positive outcomes [4]. A critical unresolved question is whether it is possible to restore muscle structure using this approach [5].
Evaluation of individuals with LBP [6, 7] and animal models [8,9,10], including models of intervertebral disc (IVD) degeneration, reveal profound structural alterations of the multifidus muscle. Although, the nature of these structural alterations differs depending on the chronicity [11] and underlying aetiology [12, 13], muscle fibrosis is a hallmark of the degenerative changes and are linked to functional outcomes [14]. Human data of the multifidus muscle in individuals with LBP displays dysregulated levels of fibrogenic genes, including collagen (Col) subtypes Col-I and -III. These changes are associated with pain duration [7] and the degree of fatty infiltration [6], respectively. Fibrogenic genes such as connective tissue growth factor (CTGF) are also dysregulated in chronic LBP [7]. Animal models of IVD degeneration (spontaneous and induced by surgical IVD lesion), show increased cross-sectional area (CSA) of Col and dysregulation of the fibrogenic genetic pathways, including increases to the expression of Col-I and -III [9, 15]. In an animal model of multifidus muscle degeneration induced by injection of glycerol into the muscle, fibrosis was associated with functional deficits and a hyperkyphotic spine deformity [14]. Degenerative structural changes to the multifidus are predictive of the worse outcomes following surgery and impair recovery in individuals suffering LBP [16, 17]. Taken together these data imply that restoration of multifidus structure is likely to be beneficial for spine health, at least for some individuals with LBP.
Physical activity, or exercise, produces beneficial outcomes in individuals with chronic LBP, including reduced pain and improved function [2]. It is plausible that the impact of exercise on back muscle structure contributes to its efficacy. In animal models of IVD degeneration, whole-body aerobic exercise reduces the immune response and fibrotic muscle changes, but not completely [9]. Effects include reduced thickening and expression of Col-I of the epimysial connective tissue that surrounds the multifidus muscle [9]. Whole-body exercise did not attenuate increased expression of Col-III [9]. An important consideration is that the back muscles are a redundant system with many unique options of muscle activation available to control and move the spine [18]. As it cannot be assumed that the multifidus muscle was activated in the whole-body exercise it is possible that incomplete restoration of muscle structure might be explained by limited activated of the multifidus muscle. A more targeted approach to multifidus muscle activation might be required achieve more complete restoration of multifidus health.
In clinical research, activation of the multifidus muscle involves voluntary exercise [2], and more recently via neurostimulation [19]. Although both reduced pain and/or disability in individuals with chronic LBP [19], it has not been tested whether multifidus fibrosis is modified. Neurostimulation enables this question to be addressed using detailed histological, immunohistochemical and gene expression techniques in an animal model. This study aimed to assess the impact of targeted activation of the multifidus, via neurostimulation, on the fibrotic alterations present in the multifidus muscle following IVD injury.
We hypothesized that following IVD injury: (1) neurostimulation of the multifidus muscle plus usual physical activity would attenuate the fibrotic changes in multifidus, compared to usual physical activity alone; (2) the greatest reduction in fibrotic changes would be present in the stimulated muscle fascicles; and (3) fibrosis might also be reduced in unstimulated, adjacent fascicles of the muscle.
Methods
Study design
A model of IVD injury shown to induce fibrosis of the multifidus [8] was used. Injury and subsequent degeneration was induced in the L1/2 and L3/4 IVD. Multifidus muscle tissue was harvested adjacent to the L2 and L4 spinous processes, which includes the major bulk of muscle fascicles that cross the injured IVD and develop fibrotic changes. To assess the impact of targeted activation of the multifidus muscle compared to usual physical activity, animals were implanted with a neurostimulation device (ReActiv8®, Mainstay Medical, and USA). The device was activated in half of the animals at three months after injury to stimulate the medial branch of the dorsal ramus at L2 to activate the fascicles of the multifidus that arise from the L2 vertebral level. No muscle fascicles of the stimulated muscle are adjacent to the L2 spinous. Adjacent to the L4 spinous process, fascicles from the L2 are located in the lateral region of the muscle (see Fig. 1). Muscle harvested at L2 includes multifidus fascicles that cross an injured IVD but are unstimulated, and at the L4 level the multifidus muscle fascicles cross an injured IVD and are stimulated. Together this allowed assessment of the impact of targeted activation of multifidus on injury-induced fibrosis in stimulated and unstimulated muscle. Tissue collected from L4 in a group of animals from a previous study with no IVD injury or implanted device were analysed as a reference for connective tissue in healthy animals.
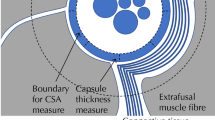
Multifidus anatomy and experimental design. Views of anatomy of the multifidus muscle fibres arising from L2 from A side and B top. Discrete muscle fascicles shown in blue (short deep fascicles), orange (superficial fascicles attaching to L4) and green (superficial fascicles attaching to L5). Arrows indicate injured intervertebral discs. All are activated by stimulation of the medial branch of the L2 dorsal ramus (black dashed line). The location stimulating electrode of the neurostimulator is shown adjacent to the dorsal ramus. C Illustration of the cross-section of multifidus adjacent to the spinous process of L2, L3 and L5 (located at the dashed lines in A) showing the approximate location of the activated muscle fascicles arising from L2 within the cross-section (blue, orange and green relate to specific fascicles shown in A). D Van Giesen’s stained multifidus cross-section from adjacent to L4 spinous process illustrating the location of the 6 regions (A–E) that the multifidus muscle used for analysis. “*” highlights the regions that include fascicles activated by neurostimulation. E Illustration of the epimysium surrounding the muscle, “macro” perimysium surrounding major muscle fascicles, “micro” perimysium surrounding muscle fibre bundles, and endomysium surrounding individual muscle fibres
Sheep and surgical procedure
Eighteen male merino sheep aged four years old were used in this study. All animals were anaesthetised in the right lateral recumbency and a left transverse retroperitoneal approach to the lateral IVD was made. A sharp full thickness linear 6 mm lesion was made to the left side of the annulus fibrosis of the L1/2 and L3/4 IVDs. The surgical site was closed routinely in layers and the sheep moved to sternal recumbency for neurostimulation device (ReActiv8, Mainstay Medical) implantation. Through a stab incision on the dorsal midline, the leads were inserted into the animal at the rostral end of the L4 spinous process and introduced with fluoroscopic guidance. Electrodes were placed bilaterally adjacent to the medial branch of the L2 dorsal ramus and were confirmed to elicit a response in the multifidus muscle with stimulation. Through a separate incision lateral and caudal to the stab incision, the battery was inserted into a pocket created adjacent to the hip. Animals were closely monitored for ten days. No complications were detected during this period.
Neurostimulation treatment
Three months after surgery the animals were randomly allocated into activation or non-activation groups. The activation group had stimulation at a frequency of 20 Hz, with a pulse width of 214 ms, and specific pulse amplitudes and electrode pairing configurations optimized to elicit to induce a palpable tonic multifidus contractions for 10 s twice per minute during the stimulation session. Activated group sheep received twice daily 30-min treatment sessions (as per the protocol found to have clinical benefit in human studies [19] until the completion of the study. The non activated group (IVD injury group) did not receive any stimulation and their devices remained inactive for the duration of the project.
Tissue collection and processing
After three months of stimulation, the animals were euthanized, and multifidus muscle tissue was collected adjacent to the spinous processes at L2 and L4. At each level, two complete cross-sections of the multifidus were harvested 5 mm cranial and caudal to the midpoint of the spinous process. The cranial section was placed in 4% paraformaldehyde overnight, and then dehydrated and embedded in paraffin. The caudal section was divided into four fascicles and each segment was snap frozen and stored at − 70 °C.
Histological stains
For histological analysis, the slides were dewaxed and rehydrated in a series of 2-min xylene, ethanol and water baths. For analysis of epimysial connective tissue that surrounds the multifidus and major macroscopic areas of perimysium surrounding discrete major muscle fascicles (Fig. 1), a Van Gieson’s stain was performed by incubating the slides in Weigert’s haematoxylin for 10 min and then washing in water before incubation in Van Gieson’s solution for 3 min and dehydrated and mounted.
The Picrosirius red stain was performed to assess microscopic areas of connective tissue of the perimysium (surrounding minor muscle fibre bundles) endomysium (surrounding individual muscle fibres) (Fig. 1). The stain was performed by submerging the rehydrated slides in Mayer’s haematoxylin for 3 min, Picrosirius red solution for 1 h before washing in acidified water and then dehydrating and mounting the slides.
Immunofluorescence assays were performed to assess the presence of Collagen -I and -III (Col-I and Col-III). Slides were washed in Tris-buffered saline (TBS) and were then treated with ethylenediaminetetraacetic acid at pH 8.0 for 45 min at 90 °C and then washed in TBS three times before incubation in 2% Bovine serum albumin (BSA) in TBS for 1 h at room temperature. Slides were then incubated overnight in Col-I (1:200, AB6308, Abcam) and Col-III (1:200, ab7778, Abcam) antibodies diluted in 2% BSA in TBS. Next, slides were washed in TBS before incubation for 1 h in room temperature with appropriate secondary antibodies. Sections were mounted with mounting media containing DAPI.
Slides were imaged using an AxioScan Z1 Scanner (Zeiss) and image analysis was performed using ImageJ (NIH). For the Van Gieson’s analysis, the entire muscle cross-section was imaged and separated into muscle, connective and adipose tissue, using the threshold function on image J. The proportion of each tissue type was determined as a proportion of total multifidus CSA. For the Picrosirius red stain and Collagen immunofluorescence assays, the multifidus muscle was divided into six regions that each include multifidus muscle fascicles that arise from different vertebral levels. This was done to gain greater insight into the impacts of neurostimulation on the different regions of the multifidus including muscle fascicles that were and were not activated by the neurostimulation (Fig. 1D). Three images at high magnification (30 times) were taken at random locations within in each region by further subdividing each region into six subregions and imaging three of the sub-regions based on a random number. All images were separated into muscle and connective tissue/collagen, using the threshold function on Image J, and the proportion of each tissue type was determined. The relative intensity of Col-I and -III staining was determined in these images using the integrated density function on Image J. The staining intensity of Col-I and -III staining in the perimysium and endomysium surrounding the muscle fibres/bundles was expressed as a proportion of the intensity of Col-I and -III staining in the dense epimysium surrounding the muscle, higher values indicate greater relative density of endomysium/perimysium connective tissue throughout the muscle. Investigators were blinded to animal group during analysis.
Muscle for control animals was available for Van Giesen’s stain and immunofluorescence assay.
Quantitative PCR (qPCR)
RNA was extracted from the deepest fascicle of the multifidus muscle at L2 and L4 using an RNeasy mini kit (QIAGEN) and reversed transcribed into cDNA (Invitrogen). qPCR analysis was performed with the IQ SYBR green master mix on a Rotor-Gene 6000 Real Time PCR machine. The following primer pairs were used; GAPDH F: 5′-CCTGGAGAAACCTGCCAAGTATG-3′ and R: 5′-GGTAGAAGAGTGAGTGTCGCTGTTG-3′; Col-I: 5′-GACATCCCACCAGTCACCTG-3′ and R: 5′-GGGACTTTGGCGTTAGGAC-3′; Col-III: 5′-GGTCAGCCTGGCGTCATGGG-3′ and R: 5′-GACCTCCAGGGCCACCTGCT-3′. The levels of gene expression were divided by the expression of housekeeping gene, GAPDH and expressed as a fold difference to the IVD injury group.
Statistical analysis
At L2, data from the Van Gieson’s analysis (total multifidus CSA, proportion of epimysial and macro perimysial connective tissue), analysis of presence of Col-I and Col-III (immunofluorescence) were compare between the Groups (Injury and Activation) with t-tests for independent samples. At L4, one way-ANOVAs were used to compare the Van Gieson’s, Col-I and Col-III relative intensity data between groups (Injury, Activation and Control). Data from the immunofluorescence analysis of presence Col-I and -III was compared between Regions (A–F) and groups (Injury, Activation and Control) using two-way repeated measures ANOVAs with a Tukey’s post-test. Data from the Picrosirius red (proportion of endomysial and micro perimysial connective tissue) was compared between regions (A–F) and groups (Injury and Activation) using a two-way repeated measures ANOVA with a Tukey’s post-test. qPCR data were compared between groups (Injury and Activation) with t-tests for independent samples. Significance was set at P < 0.05. All statistical analysis was performed using Prism 9 (GraphPad Prism).
Results
All animals survived surgery with no complications. One animal passed away prior to completion of the study following complications arising from Haemonchus contortus infection and no data from this animal was used in the study. All other animals tested negative for this intestinal parasite. Seven animals experienced superficial incisional infection at the site of battery insertion that was managed conservatively with local debridement. As comparison of data for animals with and without incisional infection with t-tests revealed no differences, all animals were included in the final analysis.
Proportion of epimysial and macro perimysial connective tissue
At L4, data from the Van Gieson’s stain (Fig. 2) revealed the proportion of the multifidus muscle composed of major areas of connective tissue distributed around and throughout in the muscle (epimysium + macro areas of perimysium) was significantly higher in the injury than control group (Fig. 2D; Table 1) but neurostimulation had no impact on total muscle CSA at either level (mean (SD) CSA-L2: Inj 104.6 (25.4) mm2; Activ 112.1 (36.3) mm2; P = 0.67; L4: Inj 101.1 (27.8) mm2; Activ. 104.5 (20.5) mm2; control 105.8 (19.9) mm2; P = 0.94). Neurostimulation of the L2 nerve root reduced the proportion this major connective tissue in the multifidus muscle following IVD Injury (Activation group), to levels that were not different to control (Fig. 2D; Table 1). Neurostimulation of L2 had no impact on the proportion of epimysial and macro perimysial connective tissue in the multifidus muscle harvested adjacent to the L2 spinous process which does not include stimulated muscle fascicles (Fig. 2B).
Epimysial and “macro” perimysium connective tissue (Van Giesen’s stain). Representative images of Van Gieson’s stained multifidus muscle at A L2 and C L4. Connective tissue is stained pink and muscle tissue in yellow. The proportions of epimysial and macro perimysium connective tissue in the cross-sectional area of the muscle at B L2 and D L4. **P < 0.01; ***P < 0.001
Proportion of endomysial and micro perimysial connective tissue
Picrosirus red staining of the microscopic perimysial and endomysial connective tissue (Fig. 3A, C) was compared for the six regions of multifidus that represent fascicles arising from different levels (Fig. 1). Activation of the multifidus by stimulation at L2 significantly reduced the connective tissue around the muscle fibres at L4 (Fig. 3D). Post hoc analysis reveal that this improvement was distributed throughout the muscle and not only in the regions that received stimulation (regions D and F) (Fig. 3D, Table 1). No differences were detected in the endomysial and micro perimysial connective tissue at L2 (Fig. 3B).
Endomysium and “micro” perimysium connective tissue (Picrosirius Red stain). Representative images of the Picrosirius Red stained at A L2 and C L4. Connective tissue is stained dark pink and muscle tissue in red. The proportions of peri/endomysial connective tissue within each of the six regions of the muscle at B L2 and D L4. *P < 0.001 for between group difference
Col-I and -III intensity (immunofluorescence) and gene expression (qPCR)
At L4, the presence of Col-I was greater for the Injury than control or activation groups (Fig. 4D; Table 1). No differences to Col-III were detected between groups for any region (Fig. 5B, D). The intensity of Col-I and -III staining of the endomysial/perimysial connective tissue relative to that of the dense epimysium was significantly less for the Activation than Injury group indicating significantly less broadly distributed connective tissue throughout the muscle (Figs. 4D, 5D, respectively; Table 1). No differences were detected between any measures of presence of collagen at L2 (Fig. 4A, B; Fig. 5A, B).
qPCR analysis detected no difference in Col-I gene expression between Injury and Activation groups at any level (Fig. 6A, B, Table 1). Neurostimulation reduced Col-III gene expression at L4 (Fig. 6B). No other differences to Col-III expression were shown (Fig. 6A).
Discussion
The results of this study confirm that IVD injury induces profound fibrotic alterations to the multifidus muscle and provide new data that this can, for most measures, be completely resolved by neurostimulation of the muscle. The study provides new data that fibrotic alterations to the multifidus following IVD injury/degeneration involve increased connective tissue at the epimysial, perimysial and endomysial levels, and that the accumulation of connective tissue is mirrored by an elevated presence of Col-I in the multifidus. The major discovery of this study is that although all animals were physically active after the IVD injury, this did not prevent accumulation of fibrosis, but complete resolution could be achieved by targeted activation of the multifidus muscle. An additional important observation was that although targeted activation produced effects on presence of Col-I in the fascicle activated by neurostimulation, its effects on connective tissue at the epimysial, perimysial and endomysial levels were also present in adjacent unstimulated areas. These observations highlight that multifidus muscle activation, in this case induced by stimulation, successfully attenuates fibrosis and this might contribute to treatment effects when applied to individuals with LBP as a plausible contribution to the reduction of pain and disability following neurostimulation.
New insights into the nature of connective tissue accumulation in the multifidus muscle
Multifidus muscle atrophy and fatty infiltration are key features of chronic LBP [11]. Investigation of the nature of fibrotic alterations to the multifidus are less clear, because of difficulties imaging connective tissue [20]. Over recent years fibrotic changes to the multifidus have been reported in individuals with chronic LBP with tissue samples harvested during surgery [6, 7] and animal models of IVD degeneration [8, 9]. The present study provides substantial clarity of gene expression and structural features of fibrosis. Data confirm that fibrotic changes present in the epimysial layer of the connective tissue in the multifidus in a model of IVD injury/degeneration. This concurs with data of increased epimysial connective tissue CSA [8] and thickness [9] in sheep and mouse models of IVD degeneration, respectively. New data extend this observation with detailed analysis of increased density of perimysial and endomysial connective tissues that make up the micro level connective tissues in the muscle.
Our new data also suggest that the increases in connective tissue are explained by increased presence of Col-I, which was significantly elevated in the Injury group. This is a novel observation and supports genetic data of increased Col-I expression in humans with chronic LBP [7] along with elevated CTGF, a fibrogenic factor that promotes fibrosis. We found little evidence of changes in presence of Col-III. Previous work has identified that alterations to Col-IIIA depend on the nature of the etiology [13] and potentially the species—animal studies of IVD injury and degeneration have shown elevated Col-I in sheep [8], and Col-III and CTGF [9] in mice models.
An important consideration is the mechanisms that might mediate the immune response that subsequently impacts multifidus muscle structure. Previous work has speculated that the immune response might be initiated by changes in muscle activation [21] or an immune response to IVD injury [22], amongst other plausible mechanisms. Although the present study cannot inform that discussion, we do provide data that stimulated muscle contraction mediates the resolution of connective tissue accumulation. This concurs with previous data that show a cytokine response in electrically stimulated muscle cells [23].
Potential consequences of multifidus muscle fibrosis
Fibrosis is likely to impact function of the multifidus muscle, and the current data provide new insight into potential consequences and a potential role in functional defects in chronic LBP [14]. First, we highlight the major role of Col-I in the fibrotic accumulation. This is potentially important as the Col-I subtype is stiffer than Col-III and could underlie the increases in passive stiffness present in the multifidus in animal models [10] and individuals with LBP [24]. In rabbits, fibrosis at 12 weeks following IVD injury and was mirrored by increases in the passive stiffness of the multifidus muscle [6]. As the ratio of Collagen I/III has been shown to be the primary predictor of muscle stiffness in muscle biopsies taken from patients with cerebral palsy [25], alterations to that ratio in the multifidus muscle could underlie the increases to passive stiffness present in patients with LBP [24]. Although enhanced passive stiffness might have positive impact on inherent stability of intervertebral segments, it could also reduce the adaptability of muscle tension that is necessary for the fitness of optimal dynamic control of the spine [26, 27] and if excessive could limit mobility and successful spine control [27]. Second, as the degree of fatty infiltration [6] is associated fibrotic alterations to the multifidus muscle, this could imply an indirect relationship with reduced capacity of muscle output.
Impact of target activation and physical activity on multifidus muscle fibrosis
Although exercise and physical activity are effective treatments for LBP [2], little is known regarding how to match the type of exercise to the individual patient. A key factor hindering optimization of exercise selection is the paucity of data revealing the mechanisms by which exercise impacts LBP, or whether the features targeted by exercise are even addressed, particularly at a molecular level. Both general aerobic exercise [28] and exercise that includes more specific back muscle activation (e.g., motor control exercise) [29] reduce pain and functional limitations in patients with chronic LBP. Recent data show that targeted activation of the multifidus via motor control exercise achieved greater improvement in multifidus muscle composition than general exercise alone [30], but both programmes reduced pain. There is debate regarding the focus on the multifidus muscle for back pain rehabilitation [31]. Although there is evidence that the multifidus muscle undergoes greater muscle changes than other back muscles in pain and injury [11], whether recovery depends on reversal of these changes has not been confirmed. A hypothesis that cannot be resolved with current data is that resolution of these changes might be critical for some individuals but not others, and tailored exercise interventions might be required. The present study cannot resolve this issue, but provides insight into how the impact of exercise on multifidus muscle health differs between general aerobic exercise and specific multifidus activation.
Earlier work has highlighted positive but incomplete effects of whole-body aerobic exercise on multifidus muscle fibrosis. A study of knock-out mice that develop spontaneously develop IVD degeneration, showed aerobic exercise induced incomplete attenuation of the IVD degeneration dependent increase in epimysial thickness [9], decreased Col-I [9], but no effect on degeneration dependent increase in Col-III [9]. Although benefits were identified, aerobic exercise did not fully attenuate the fibrotic changes and restore the structure of the multifidus. In contrast, in the present study, targeted activation of the multifidus muscle appeared to completely attenuate the IVD injury-dependent alterations to the structure of the multifidus muscle, and this was apparent in the dense epimysium as well as the perimysium and endomysium throughout the muscle. Targeted activation also appeared to maintain the composition of the connective tissue. These could underlie the increased effectiveness of motor control exercises in reducing pain and improving multifidus composition compared to general aerobic exercise, which is reported in clinical studies [30]. Further, as stimulation was only commenced three months after the IVD injury, when muscle structural changes would have already commenced [8], these data suggest specific muscle activation has potential to reverse fibrotic changes. Whether, long standing changes can be reversed remains unresolved. Taken together these data suggest specific muscle activation has advantages over general whole-body exercise when the objective is to maintain multifidus muscle structural health.
Neurostimulation as an alternative to exercise
Restorative neurostimulation is an emerging therapy in the treatment of LBP [32]. Clinical studies have shown significant improvements in pain, quality of life and disability, in individuals with chronic refractory LBP [19]. The precise mechanisms that promote these clinical effects are currently unknown. The significant impact of targeted activation, via neurostimulation, on fibrosis in the multifidus muscle provides a potential candidate mechanism via improvement of the mechanical capacity of multifidus to regulate loading on spinal tissues. As expected, neurostimulation produced effects on the fibrotic alterations in the stimulated fascicles of the multifidus and also in adjacent regions. As the neurostimulation device is implanted routinely to stimulate the dorsal ramus at L2, and fascicles innervated by that segment only comprise a small region of the cross-section of multifidus at the lower lumbar levels where spine pathology is more common, it was critical to investigate whether positive effects of stimulation on fibrosis could also impact adjacent unstimulated muscle fascicles. For most measures this was convincingly identified and indicates that the effect of targeted activation extends beyond on the activated fascicles. The mechanisms to explain this effect is not resolved but might relate to an impact of muscle activation on the immune system. Previous work has highlighted a role of the dysregulation of the immune system, including an upregulation of the pro-inflammatory factors (increased expression of interleukin 1β and tumour necrosis factor) on fibrosis [8, 21, 33] and exercise is known to moderate such a response [21].This response might easily spread to adjacent muscle areas, providing a plausible mechanism to explain the clinical effectiveness of neurostimulation therapy in chronic LBP. If such “spreading” of the effect of exercise explains the impact of stimulation on adjacent non-stimulated muscle regions it might be speculated that spreading of immune response should have been possible with whole-body exercise. Yet this was not observed—general aerobic exercise reduced the pro-inflammatory response in the multifidus in a model of IVD degeneration but with only a partial resolution of the fibrotic changes. A plausible explanation is that different types and intensities of exercise are linked with different inflammatory profiles in clinical studies [34]. It is reasonable to speculate that targeted neurostimulation would promoting an inflammatory response that differs from whole-body aerobic exercise, and that this different response induces the more complete resolution of the fibrotic changes. Gaining insights into the difference in the inflammatory response in the multifidus between general aerobic exercise and targeted activation, via neurostimulation or specific muscle exercise, could be key to understanding the increased clinical benefits of these different approaches to rehabilitation [30].
Methodological issues
Interpretation of the data requires consideration of several methodological issues. First, as noted above, several animals developed incisional infections associated with the battery site, although this might have induced an augmented immune response and impacted the fibrosis, additional analyses confirmed findings remained despite removal of this group. Second, the use of data from a separate group of animals as controls is not ideal. Animals were age and species matched, but differences could have been present in the processing of the tissue that should be considered when interpreting the results (e.g. dissection of the multifidus immediately [present study] or several hours [15] after euthanasia which might impact analyses). Third, translational of these results to clinical studies should consider the difference in structure of the multifidus muscle between sheep and humans, such as the difference in predominate muscle fibre types between the species. In support of the translation of these findings is evidence that similar muscle and immune responses are present in humans with disc pathology [35, 36].
Conclusion
This study has provided new insights into the fibrotic processes present in a model of IVD injury and degeneration. The attenuation of these changes following targeted activation of the multifidus, provides new insights into the treatment of LBP.
References
Global Burden of Disease 2021 Low Back Pain Collaborators (2023) Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol 5:e316–e329. https://doi.org/10.1016/s2665-9913(23)00098-x
Hayden JA, Ellis J, Ogilvie R, Malmivaara A, van Tulder MW (2021) Exercise therapy for chronic low back pain. Cochrane Database Syst Rev 9:CD009790. https://doi.org/10.1002/14651858.CD009790.pub2
Wun A, Kollias P, Jeong H, Rizzo RR, Cashin AG, Bagg MK, McAuley JH, Jones MD (2021) Why is exercise prescribed for people with chronic low back pain? A review of the mechanisms of benefit proposed by clinical trialists. Musculoskelet Sci Pract 51:102307. https://doi.org/10.1016/j.msksp.2020.102307
Sipaviciene S, Kliziene I (2020) Effect of different exercise programs on non-specific chronic low back pain and disability in people who perform sedentary work. Clin Biomech (Bristol, Avon) 73:17–27. https://doi.org/10.1016/j.clinbiomech.2019.12.028
Pinto SM, Boghra SB, Macedo LG, Zheng YP, Pang MYC, Cheung JPY, Karppinen J, Samartzis D, Wong AYL (2021) Does motor control exercise restore normal morphology of lumbar multifidus muscle in people with low back pain?—A systematic review. J Pain Res 14:2543–2562. https://doi.org/10.2147/jpr.S314971
Anderson B, Ordaz A, Zlomislic V, Allen RT, Garfin SR, Schuepbach R, Farshad M, Schenk S, Ward SR, Shahidi B (2022) Paraspinal muscle health is related to fibrogenic, adipogenic, and myogenic gene expression in patients with lumbar spine pathology. BMC Musculoskelet Disord 23:608. https://doi.org/10.1186/s12891-022-05572-7
Shahidi B, Fisch KM, Gibbons MC, Ward SR (2020) Increased fibrogenic gene expression in multifidus muscles of patients with chronic versus acute lumbar spine pathology. Spine (Phila Pa 1976) 45:E189-e195. https://doi.org/10.1097/brs.0000000000003243
Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, Little C, Melrose J (2015) Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine (Phila Pa 1976) 40:1057–1071. https://doi.org/10.1097/brs.0000000000000972
James G, Klyne DM, Millecamps M, Stone LS, Hodges PW (2019) ISSLS prize in Basic science 2019: physical activity attenuates fibrotic alterations to the multifidus muscle associated with intervertebral disc degeneration. Eur Spine J 28:893–904. https://doi.org/10.1007/s00586-019-05902-9
Brown SH, Gregory DE, Carr JA, Ward SR, Masuda K, Lieber RL (2011) ISSLS prize winner: Adaptations to the multifidus muscle in response to experimentally induced intervertebral disc degeneration. Spine (Phila Pa 1976) 36:1728–1736. https://doi.org/10.1097/BRS.0b013e318212b44b
Hodges PW, Danneels L (2019) Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther 49:464–476. https://doi.org/10.2519/jospt.2019.8827
Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, Van Oosterwijck J, Dhondt E, Danneels L (2017) Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J 17:1285–1296. https://doi.org/10.1016/j.spinee.2017.04.025
Ordaz A, Anderson B, Zlomislic V, Allen RT, Garfin SR, Schuepbach R, Farshad M, Schenk S, Ward SR, Shahidi B (2023) Paraspinal muscle gene expression across different aetiologies in individuals undergoing surgery for lumbar spine pathology. Eur Spine J 32:1123–1131. https://doi.org/10.1007/s00586-023-07543-5
Noonan AM, Buliung E, Briar KJ, Quinonez D, Séguin CA, Brown SHM (2023) Glycerol induced paraspinal muscle degeneration leads to hyper-kyphotic spinal deformity in wild-type mice. Sci Rep 13:8170. https://doi.org/10.1038/s41598-023-35506-9
Hodges PW, James G, Blomster L, Hall L, Schmid AB, Shu C, Little C, Melrose J (2014) Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine (Phila Pa 1976) 39:1010–1017. https://doi.org/10.1097/brs.0000000000000318
Storheim K, Berg L, Hellum C, Gjertsen Ø, Neckelmann G, Espeland A, Keller A (2017) Fat in the lumbar multifidus muscles—predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC Musculoskelet Disord 18:145. https://doi.org/10.1186/s12891-017-1505-5
Jermy JE, Copley PC, Poon MTC, Demetriades AK (2020) Does pre-operative multifidus morphology on MRI predict clinical outcomes in adults following surgical treatment for degenerative lumbar spine disease? A systematic review. Eur Spine J 29:1318–1327. https://doi.org/10.1007/s00586-020-06423-6
Tier L, Salomoni SE, Hug F, Besomi M, Hodges PW (2023) Adaptability of the load sharing between the longissimus and components of the multifidus muscle during isometric trunk extension in healthy individuals. Eur J Appl Physiol 123:1879–1893. https://doi.org/10.1007/s00421-023-05193-5
Gilligan C, Volschenk W, Russo M, Green M, Gilmore C, Mehta V, Deckers K, De Smedt K, Latif U, Georgius P, Gentile J, Mitchell B, Langhorst M, Huygen F, Baranidharan G, Patel V, Mironer E, Ross E, Carayannopoulos A, Hayek S, Gulve A, Van Buyten JP, Tohmeh A, Fischgrund J, Lad S, Ahadian F, Deer T, Klemme W, Rauck R, Rathmell J, Maislin G, Heemels JP, Eldabe S (2023) Long-term outcomes of restorative neurostimulation in patients with refractory chronic low back pain secondary to multifidus dysfunction: two-year results of the reactiv8-b pivotal trial. Neuromodulation 26:87–97. https://doi.org/10.1016/j.neurom.2021.10.011
Carlier PG, Marty B, Scheidegger O, Loureiro de Sousa P, Baudin PY, Snezhko E, Vlodavets D (2016) Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis 3:1–28. https://doi.org/10.3233/jnd-160145
James G, Millecamps M, Stone LS, Hodges PW (2018) Dysregulation of the inflammatory mediators in the multifidus muscle after spontaneous intervertebral disc degeneration sparc-null mice is ameliorated by physical activity. Spine (Phila Pa 1976) 43:E1184-e1194. https://doi.org/10.1097/brs.0000000000002656
James G, Blomster L, Hall L, Schmid AB, Shu CC, Little CB, Melrose J, Hodges PW (2016) Mesenchymal stem cell treatment of intervertebral disc lesion prevents fatty infiltration and fibrosis of the multifidus muscle, but not cytokine and muscle fiber changes. Spine (Phila Pa 1976) 41:1208–1217. https://doi.org/10.1097/brs.0000000000001669
Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, de Angelis MH, Häring HU, Weigert C (2013) Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol 305:C877-886. https://doi.org/10.1152/ajpcell.00043.2013
Koppenhaver S, Gaffney E, Oates A, Eberle L, Young B, Hebert J, Proulx L, Shinohara M (2020) Lumbar muscle stiffness is different in individuals with low back pain than asymptomatic controls and is associated with pain and disability, but not common physical examination findings. Musculoskelet Sci Pract 45:102078. https://doi.org/10.1016/j.msksp.2019.102078
Smith LR, Pichika R, Meza RC, Gillies AR, Baliki MN, Chambers HG, Lieber RL (2021) Contribution of extracellular matrix components to the stiffness of skeletal muscle contractures in patients with cerebral palsy. Connect Tissue Res 62:287–298. https://doi.org/10.1080/03008207.2019.1694011
Reeves NP, Everding VQ, Cholewicki J, Morrisette DC (2006) The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res 174:694–700. https://doi.org/10.1007/s00221-006-0516-5
Reeves NP, Cholewicki J (2013) Spine systems science: a primer on the systems approach. In: Hodges PW, Cholewicki J, van Dieen J (eds) Spinal control: the rehabilitation of back pain. Elsevier, Amsterdam
Meng XG, Yue SW (2015) Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil 94:358–365. https://doi.org/10.1097/phm.0000000000000188
Wang XQ, Zheng JJ, Yu ZW, Bi X, Lou SJ, Liu J, Cai B, Hua YH, Wu M, Wei ML, Shen HM, Chen Y, Pan YJ, Xu GH, Chen PJ (2012) A meta-analysis of core stability exercise versus general exercise for chronic low back pain. PLoS ONE 7:e52082. https://doi.org/10.1371/journal.pone.0052082
Fortin M, Rye M, Roussac A, Montpetit C, Burdick J, Naghdi N, Rosenstein B, Bertrand C, Macedo LG, Elliott JM, Dover G, DeMont R, Weber MH, Pepin V (2023) The effects of combined motor control and isolated extensor strengthening versus general exercise on paraspinal muscle morphology, composition, and function in patients with chronic low back pain: a randomized controlled trial. J Clin Med 12(18):5920. https://doi.org/10.3390/jcm12185920
Matheve T, Hodges P, Danneels L (2023) The role of back muscle dysfunctions in chronic low back pain: state-of-the-art and clinical implications. J Clin Med 12(17):5510. https://doi.org/10.3390/jcm12175510
Tieppo Francio V, Westerhaus BD, Carayannopoulos AG, Sayed D (2023) Multifidus dysfunction and restorative neurostimulation: a scoping review. Pain Med. https://doi.org/10.1093/pm/pnad098
James G, Sluka KA, Blomster L, Hall L, Schmid AB, Shu CC, Little CB, Melrose J, Hodges PW (2018) Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J 27:1744–1756. https://doi.org/10.1007/s00586-018-5652-7
Ayari S, Abellard A, Carayol M, Guedj É, Gavarry O (2023) A systematic review of exercise modalities that reduce pro-inflammatory cytokines in humans and animals’ models with mild cognitive impairment or dementia. Exp Gerontol 175:112141. https://doi.org/10.1016/j.exger.2023.112141
James G, Chen X, Diwan A, Hodges PW (2021) Fat infiltration in the multifidus muscle is related to inflammatory cytokine expression in the muscle and epidural adipose tissue in individuals undergoing surgery for intervertebral disc herniation. Eur Spine J 30:837–845. https://doi.org/10.1007/s00586-020-06514-4
Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, Garfin SR, Ward SR (2017) Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 35:2700–2706. https://doi.org/10.1002/jor.23597
Acknowledgements
The authors acknowledge all the staff at the QASP facility who cared for the animals.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by Mainstay Medical and the National Health and Medical Research Council of Australia (1091302; 1194937).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Greg James], and [Paul W. Hodges]. The surgeries were performed by [Ben Ahern] and [Wendy Goodwin]. The first draft of the manuscript was written by [Greg James] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Ben Goss is employed by Mainstay Medical. The study received funding from Mainstay Medical. The remaining authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
James, G., Ahern, B.J., Goodwin, W. et al. Targeted multifidus muscle activation reduces fibrosis of multifidus muscle following intervertebral disc injury. Eur Spine J 33, 2166–2178 (2024). https://doi.org/10.1007/s00586-024-08234-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08234-5