Abstract
Background
Patients with lumbar spinal stenosis (LSS) sometimes have lower lumbar lordosis (LL), and the incidence of LSS correlates closely with the loss of LL. The few studies that have evaluated the association between LL and clinical outcomes after non-instrumented surgery for LSS show conflicting results. This study investigates the association between preoperative LL and changes in PROMs 2 years after decompressive surgery.
Method
This prospective cohort study obtained preoperative and postoperative data for 401 patients from the multicenter randomized controlled spinal stenosis trial as part of the NORwegian degenerative spondylolisthesis and spinal STENosis (NORDSTEN) study. Before surgery, the radiological sagittal alignment parameter LL was measured using standing X-rays. The association between LL and 2-year postoperative changes was analyzed using the oswestry disability index (ODI), a numeric rating scale (NRS) for low back and leg pain, the Zurich claudication questionnaire (ZCQ), and the global perceived effect (GPE) score. The changes in PROMs 2 years after surgery for quintiles of lumbar lordosis were adjusted for the respective baseline PROMs: age, sex, smoking, and BMI. The Schizas index and the Pfirrmann index were used to analyze multiple regressions for changes in PROMs.
Results
There were no associations in the adjusted and unadjusted analyses between preoperative LL and changes in ODI, ZCQ, GPE, and NRS for back and leg pain 2 years after surgery.
Conclusion
LL before surgery was not associated with changes in PROMs 2 years after surgery. Lumbar lordosis should not be a factor when considering decompressive surgery for LSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative changes in the lumbar spine such as bulging of the intervertebral disk and hypertrophy of the facet joints and ligaments can result in narrowing of the spinal canal and lumbar spinal stenosis (LSS). The clinical presentation of patients with LSS is generally leg pain and numbness when walking, so-called neurogenic claudication [1]. Leaning forward often relieves the symptoms as the cross-sectional area of the spinal canal increases and the compression of the neurovascular structures decreases [2]. In addition, degenerative changes in the lumbar spine result in closer contact with the spinal processes; if there is a direct contact with the spinal processes, the lordosis in that segment will decrease [3]. Changes in posture activate compensatory mechanisms such as the retroversion of the pelvis, the extension of the hips, reduction of lumbar lordosis (LL), bending of the knees, and increase of the sagittal vertical axis (SVA) [4]. Patients with lumbar spinal stenosis have been reported to have lower LL compared to a control group (i.e., no lumbar spinal stenosis symptoms) [5]. The incidence of LSS correlates closely with the loss of LL, which may be related to both adaptive pain-relieving forward leaning and degenerative spinal changes such as degeneration and reduction of disk heights and age-related muscle atrophy [6]. Some studies have found an association between LL before and outcomes after decompressive surgery for lumbar spinal stenosis [7]. However, most previous studies have included few patients and do not adjust for the association between lumbar lordosis and outcomes for other variables that may impact outcomes after surgery. Thus, little is known about the association between LL and clinical outcomes after non-instrumented surgery for lumbar spinal stenosis. This study investigates the association between LL before surgery and changes in patient-related outcome measures 2 years after minimally invasive surgery for lumbar spinal stenosis.
Materials and methods
Study population
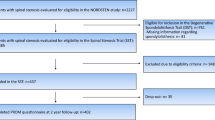
This prospective cohort study is based on data from a multicenter randomized controlled spinal stenosis trial (SST) from the NORwegian degenerative spondylolisthesis and spinal STENosis (NORDSTEN) study, which included patients with LSS without degenerative spondylolisthesis. The inclusion and exclusion criteria for the NORDSTEN-SST are presented in Table 1. The patients underwent non-instrumented decompression spinal surgery with minimally invasive surgical procedures, i.e., unilateral laminotomy with crossover (UL), bilateral laminotomy (BL), or spinous process osteotomy (SPO). The NORDSTEN-SST is well described in previous publications [1, 8,9,10] and registered in ClinicalTrials.gov under the identifier NCT02007083. A flow chart of the study cohort is presented in Fig. 1. At baseline, information about age, gender, body mass index (BMI), and smoking as well as Schizas scores, Pfirrmann scores, ODI, ZCQ, and NRS for leg and back pain was collected.
Preoperative and postoperative radiological imaging
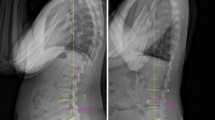
LL was measured using standing lateral X-rays of the lumbar region at the intersection between the line ending from the upper endplate of L1 and the other extending from the upper endplate of S1 (Fig. 2). All radiological images were imported and stored in a picture and archiving system (PACS), Sectra IDS7 Sweden, and the integrated software tools for angle measurements were used.
Outcome assessment and patient-reported outcome measures
Patient-reported outcome measures (PROMs) used in this study were self-administered at admission for surgery (baseline) and 2 years after surgery. The primary outcome was changed in pain-related physical function and assessed by the oswestry disability index (ODI) questionnaire, from baseline to the 2-year follow-up. Secondary outcomes were changes in the Zurich claudication questionnaire (ZCQ), a self-administered instrument used to evaluate symptom severity, physical function, and surgical satisfaction in lumbar spinal stenosis. The ZCQ score includes values for physical function and symptom severity. Furthermore, changes in NRS back pain and leg pain were registered. At the 2-year follow-up, a 7-point global perceived effect (GPE) scale and questionnaire was used to collect data.
Statistical analysis
Descriptive statistics for continuous variables are presented as means with standard deviations, and categorical data are presented as numbers and percentages. The total cohort was divided into quintiles based on the preoperative LL and analyzed in relation to changes in PROMs 2 years after surgery. The mean changes in PROMs, ODI, ZCQ, GPE, and NRS for back and leg pain are presented with means with a 95% confidence interval (CI). At baseline, groups were compared using the ANOVA test for continuous variables and the Chi-square test for categorical variables. The quintiles were compared with a likelihood ratio test in relation to changes in PROMs 2 years after surgery. The changes in proms 2 years after surgery in relation to quintiles of lumbar lordosis were adjusted for respective baseline PROMs and for age, sex, smoking, BMI, Schizas score, and Pfirrmann score. Multiple regressions were also used to analyze the association between baseline parameters and clinical outcomes 2 years after surgery. The variables in the regression model were baseline PROMS, age, sex, BMI, Schizas score, and Pfirrmann score. A p < 0.05 was significant. We used SPSS (IBM SPSS Statistics for Mac, Version 26.0, Armonk, NY: IBM Corp. USA) for statistical analyses.
Results
Demographic characteristics
The patient characteristics for the quintiles are presented in Table 2. A total of 437 patients are included in the NORDSTEN-SST study; 401 had complete radiology examinations with standing X-rays before surgery and therefore were included in this study. The mean patient age was 67 years (8.2 SD). The study included 208 male and 193 female patients. Of the 401 patients included in this study, 314 were non-smokers, 81 (20%) were smokers, and six had missing information about smoking. The mean BMI before surgery was 27.7 (SD 4.2). There were no statistical differences between the clinical characteristics of the groups at baseline except for patients in the middle quintiles of lumbar lordosis (Q2 and Q3), who had less leg pain before surgery (p = 0.04).
Changes in PROMs 2 years after surgery
The patients included in this analysis showed significant improvements from baseline to the 2-year follow-up after surgery with a mean change for ODI of − 1.8 (CI − 17.1 ‒ − 21); for ZCQ symptom score, the change was − 2.3 (CI − 2.2 ‒ − 2.4); for ZCQ physical function score, the change was − 1.7 (− 1.6 ‒ − 1.7); for NRS for back pain, the change was − 3.6 (− 3.3 ‒ − 3.9); and for NRS leg pain, the change was − 2.9 (− 2.7 ‒ − 3.2). These results agree with previously reported results. [8]
Association between lumbar lordosis and changes in PROMs 2 years after surgery
There were no statistically significant differences between the quintiles of LL in relation to changes in outcomes 2 years after surgery for ODI (p = 0.51, adj p = 0.18), ZQC symptom score (p = 0.40, adj p = 0.24), ZCQ function score (p = 0.37, adj p = 0.40), NRS back pain (p = 0.93, adj p = 0.68), NRS leg pain (p = 0.52, adj p = 0.38), and GPE (p = 0.26, adj p = 0.10) (Table 3).
Patients in the middle quintile (45.3–53 degrees) of lumbar lordosis had significantly higher changes in ZCQ symptom score (p = 0.024) and GPE (p = 0.022). No other quintiles were associated with changes in PROMs 2 years after surgery. All baseline PROMs, BMI, and smoking were associated with significant changes in the multiple regression analysis (Supplementary 1).
Discussion
In this prospective cohort study, no significant associations were found for the degree of the LL before surgery and changes in PROMs 2 years after surgery.
As the prevalence of lumbar spinal stenosis is increasing due to an aging population and lumbar decompressive surgery without instrumentation is the first choice of surgical treatment for these patients [11], identification of variables that can predict less favorable outcomes after decompression surgery is important. Because the incidence of LSS in adults correlates with the loss of LL [6], it is necessary to evaluate the association of preoperative LL with outcomes after non-instrumentation decompression surgery. Sagittal alignment measurements of radiographic parameters, such as high LL, have been demonstrated to be associated with less back pain in patients with spinal deformities and lumbar spinal stenosis [12, 13]. Furthermore, a possible restoration of a low LL after decompressive surgery may be important for the maintenance of the overall sagittal balance [14]. However, the relationship between LL and outcomes after decompressive surgery for lumbar spinal stenosis patients has been sparsely studied, and the few studies that have studied this relationship have produced contradictory findings [7, 15, 16]. Chang et al. [15] found a correlation between small lumbar lordosis and poor postoperative physical score and VAS score in a prospective cohort of 85 patients using a linear mixed effect model without adjusting for covariation factors. Similar findings were reported by Costa et al. [16]. They divided their 104 patients into two groups by the LL 50th percentile and found a weak but statistically significant correlation between a small LL before surgery and ODI 1 year after surgery. However, Mirzashahi et al. [7] reported that a lower LL was associated with a better postoperative improvement for VAS and ODI in spinal stenosis patients, but the association between small LL and PROMs found in the regression analyses was only reported for the subgroup of patients who underwent a fusion during decompressive surgery [7].
To evaluate the association between LL and outcomes in a systematic way, we divided our patient cohort into quintiles based on preoperative LL. We found no association between the patients within these quintiles and the PROMs evaluated 2 years after surgery, indicating that preoperative LL does not have any statistically significant effect on postoperative outcome for patients with spinal stenosis undergoing decompressive surgery.
Unlike most other studies, our study included a large number of patients, which allowed us to adjust for other variables in the analyses. Furthermore, in the adjusted analyses, no associations between the quintiles of lumbar lordosis and improvements 2 years after decompressive surgery were detected. However, a small but significant association was found between patients in the third quintile (Q3) and changes in ZCQ symptom score as well as GPE in the multiple regression model. Even when these associations were significant, they were not clinically relevant.
The degree of lumbar lordosis before surgery was associated with leg pain at baseline in a somewhat unexpected way as the patients in the lowest quintile (Q1) and the two quintiles with the highest lumbar lordosis (Q4 and Q5) reported more leg pain before surgery compared to the two quintiles in the middle (Q2 and Q3). We do not have any clinical reasonable explanation for this observation, although it may have a biomechanical explanation or just be coincidental.
The evaluation of sagittal balance, including lumbar lordosis, with a standing X-ray before surgery has previously been shown to be significant for patients with spinal stenosis and degenerative deformities [12, 13]. This has raised both attention and discussion about the needs for preoperative evaluation of sagittal balance and lumbar lordosis also in patients without any clear deformity or clinical imbalance. Our study, however, suggests that there is no need to pay attention to the degree of the lordosis in non-deformity patients with spinal stenosis undergoing decompressive surgery. It needs to be remembered that this study has limited the evaluation of the effect of preoperative LL on outcomes 2 years after surgery. It remains to be investigated whether changes of lumbar lordosis after decompressive surgery, such as a restoration of a previously higher lordosis, influence patient-related outcomes.
Strengths and limitations
The main strength of this study is its large sample size and structured study protocol. To our knowledge, this study is the largest study of patients undergoing non-instrumented decompressive surgery for lumbar spinal stenosis that investigates the association between LL before surgery and postoperative outcomes. As this study has a cross-sectional design, the reason for a low LL is totally unknown: it may be due to patient phenotypes or acquired changes as the result of degeneration and spinal stenosis. The NORDSTEN-SST collected data using standing lumbar spine X-rays; however, full spine standing X-rays were not performed, so the overall sagittal balance could not be evaluated. Moreover, the patients in the cohort were operated on using three minimal invasive surgical techniques, which showed similar results in the full NORDSTEN-SST cohort [8].
Conclusion
The degree of LL before surgery was not associated with changes in PROMs 2 years after surgery. Lumbar lordosis should not be a factor when considering decompressive surgery for LSS.
Ethics and trial registration
The Committee for Medical and Health Research Ethics of Central Norway approved the study (study identifier: 2011/2034). The study was registered at ClinicalTrials.gov on November 22, 2013, under the identifier NCT02007083. All patients provided written informed consent.
References
Austevoll IM et al (2019) Decompression alone versus decompression with instrumental fusion the NORDSTEN degenerative spondylolisthesis trial (NORDSTEN-DS); study protocol for a randomized controlled trial. BMC Musculoskeletal Disorder 20(1):7. https://doi.org/10.1186/s12891-018-2384-0
Roussouly P et al (2005) Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine 30(3):346–353. https://doi.org/10.1097/01.brs.0000152379.54463.65
Aylott CEW et al (2012) Spinous process morphology: the effect of ageing through adulthood on spinous process size and relationship to sagittal alignment. Eur Spine J 21:1007–1012. https://doi.org/10.1007/s00586-011-2029-6
Bouknaitir JB et al (2022) Change in sagittal alignment after decompression alone in patients with lumbar spinal stenosis without significant deformity: a prospective cohort study. J Neurosurg Spine 37(1):57–63. https://doi.org/10.3171/2021.10.SPINE21445
Abbas J et al (2010) Degenerative lumbar spinal stenosis and lumbar spine configuration. Eur Spine J 19(11):1865–1873. https://doi.org/10.1007/s00586-010-1516-5
Madkouri R et al (2018) Improvement in sagittal balance after decompression surgery without fusion in patients with degenerative lumbar stenosis: clinical and radiographic results at 1 Year. World Neurosurg 114:e417–e424. https://doi.org/10.1016/j.wneu.2018.03.002
Mirzashahi B et al (2022) Factors affecting the outcome of lumbar canal stenosis surgery: a two-year follow-up study. Caspian J Neurol Sci 8(3):143–148. https://doi.org/10.32598/CJNS.8.30.4
Hermansen E et al (2022) Comparison of 3 different minimally invasive surgical techniques for lumbar spinal stenosis: a randomized clinical trial. JAMA Netw Open 5(3):e224291. https://doi.org/10.1001/jamanetworkopen.2022.4291.PMID:35344046;PMCID:PMC8961320
Hermansen E, Austevoll IM, Romild UK et al (2017) Study-protocol for a randomized controlled trial comparing clinical and radiological results after three different posterior decompression techniques for lumbar spinal stenosis: the Spinal Stenosis Trial (SST) (part of the NORDSTEN Study). BMC Musculoskelet Disord 18:121
Indrekvam K, Bånerud IF, Hermansen E et al (2023) The Norwegian degenerative spondylolisthesis and spinal stenosis (NORDSTEN) study: study overview, organization structure and study population. Eur Spine J 32:4162–4173
Ogen I et al (2022) Factors associated with low back pain in patients with lumbar spinal stenosis: a cross-sectional study. BMC Musculoskelet Disord 23:552. https://doi.org/10.1186/s12891-022-05483-7
Hatakka J et al (2021) Effect of lumbar laminectomy on spinal sagittal alignment: a systematic review. Eur Spine J 30:2413–2426. https://doi.org/10.1007/s00586-021-06827-y
Dohzono S et al (2016) Factors associated with improvement in sagittal spinal alignment after microendoscopic laminotomy in patients with lumbar spinal canal stenosis. J Neurosurg Spine 25:39–45. https://doi.org/10.3171/2015.12.SPINE15805
Chun SW et al (2017) The relationships between low back pain and lumbar lordosis: a systematic review and meta-analysis. Spine J 17:1180–1191
Chang HS et al (2018) Influence of lumbar lordosis on the outcome of decompression surgery for lumbar canal stenosis. World Neurosurg 109:e684–e690. https://doi.org/10.1016/j.wneu.2017.10.055
Costa MA et al (2021) Correlation between clinical outcomes and spinopelvic parameters in patients with lumbar stenosis undergoing decompression surgery. Eur Spine J 30(2):1–8
Schizas C et al (2010) Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 35(21):1919–1924. https://doi.org/10.1097/brs.0b013e3181d359bd
Pfirrmann CW et al (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26:1873–1878
Funding
Open access funding provided by Umea University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wänman, J., Åkerstedt, J., Banitalebi, H. et al. The association between lumbar lordosis preoperatively and changes in PROMs for lumbar spinal stenosis patients 2 years after spinal surgery: radiological and clinical results from the NORDSTEN-spinal stenosis trial. Eur Spine J 33, 1950–1956 (2024). https://doi.org/10.1007/s00586-024-08137-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08137-5