Abstract
Purpose
As an important treatment for spinal metastasis, surgery has strict applicable conditions. Although various organizations have formulated different guidelines on surgical treatment for spinal metastasis (SM), there are certain differences in the content, standardization and quality of the guidelines and it is necessary to make a critical appraisal of them. We aim to systematically review and appraise the current guidelines on surgical treatments of SM and summarize the related recommendations with the quality evaluation of supporting evidence, as to provide a reference for the standardization of surgical treatment plans, and help clinical front-line medical workers can make safe and effective clinical decisions faster.
Methods
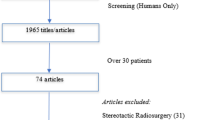
We searched Pubmed, Web of Science, and Embase for three major databases and online guideline databases. According to certain inclusion and exclusion criteria, the latest guidelines on the surgical treatment of SM were sorted out. AGREE II was used to evaluated the guideline’s quality, and we extracted and compared the recommended treatment content of each guideline with evaluating by the evidence-grading scale.
Results
Eight guidelines from 2013 to 2019 were included. Seven guidelines are comprehensive guidelines and one related to the reconstructive surgery of SM. Five guidelines were evaluated as “recommended,” and three guidelines were evaluated as “recommended with modifications.” Regarding the indications of surgery with SM, four guidelines, seven guidelines, seven guidelines, three guidelines and three guidelines recommended surgical treatment for patients with SM with intractable pain, mechanical instability, metastatic epidural spinal cord compression (MESCC), recurrent spinal metastasis (RSM), and survival predication, respectively. Regarding the surgical strategies, three guidelines recommended minimally invasive therapy but had strict indications. Six guidelines and five guidelines recommend palliative surgery and with receiving radiation therapy, respectively. For the aggressive surgery, only one guideline recommended to apply to patients in good general conditions who has isolated symptomatic SM. Regarding the surgical reconstructions, one guideline didn’t recommend iliac bone graft and three guidelines recommended PMMA bone cement.
Conclusion
Most of the guidelines do not provide clear criteria for surgical application and provide more of a basic framework. The level of evidence for these surgical recommendations ranges from LOE B to D, and almost all guidelines recommend vertebroplasty and kyphoplasty, but for palliative and more aggressive surgery, which recommended to personalize specific surgical strategies with multidisciplinary collaboration.



Similar content being viewed by others
Data availability
All supporting data are available from the corresponding author upon reasonable request.
References
Yin M, Sun Z, Ding X, Wang T, Sun Y, Li L, Gao X, Ma J, Huang Q, Xiao J, Mo W (2022) Cross-cultural adaptation and validation of simplified Chinese version of the spine oncology study group outcomes questionnaire (SOSGOQ) 2.0 with its assessment in clinical setting. Spine J 22:2024–2032. https://doi.org/10.1016/j.spinee.2022.08.013
Schoenfeld AJ, Ferrone ML, Blucher JA, Agaronnik N, Nguyen L, Tobert DG, Balboni TA, Schwab JH, Shin JH, Sciubba DM, Harris MB (2022) Prospective comparison of the accuracy of the new England spinal metastasis score (NESMS) to legacy scoring systems in prognosticating outcomes following treatment of spinal metastases. Spine J 22:39–48. https://doi.org/10.1016/j.spinee.2021.03.007
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Ryu S, Deshmukh S, Timmerman RD, Movsas B, Gerszten P, Yin FF, Dicker A, Abraham CD, Zhong J, Shiao SL, Tuli R, Desai A, Mell LK, Iyengar P, Hitchcock YJ, Allen AM, Burton S, Brown D, Sharp HJ, Dunlap NE, Siddiqui MS, Chen TH, Pugh SL, Kachnic LA (2023) Stereotactic radiosurgery versus conventional radiotherapy for localized vertebral metastases of the Spine: phase 3 results of NRG oncology/RTOG 0631 randomized clinical trial. JAMA Oncol 9:800–807. https://doi.org/10.1001/jamaoncol.2023.0356
Sciubba DM, Pennington Z, Colman MW, Goodwin CR, Laufer I, Patt JC, Redmond KJ, Saylor P, Shin JH, Schwab JH, Schoenfeld AJ, Committee NSO (2021) Spinal metastases 2021: a review of the current state of the art and future directions. Spine J 21:1414–1429. https://doi.org/10.1016/j.spinee.2021.04.012
Cao X, Jiang W, Zhang B, Zhao X, Yu H, Lei M, Cao Y, Su X, Liu Y (2023) A new treatment strategy for spinal metastasis: the “systemic conditions, effectiveness of systemic treatment, neurology, and oncology” decision framework system. Neurosurgery. https://doi.org/10.1227/neu.0000000000002709
Chiu RG, Mehta AI (2020) Spinal metastases. JAMA 323:2438. https://doi.org/10.1001/jama.2020.0716
Chakravarthy VB, Laufer I, Amin AG, Cohen MA, Reiner AS, Vuong C, Persaud PS, Ruppert LM, Puttanniah VG, Afonso AM, Tsui VS, Brallier JW, Malhotra VT, Bilsky MH, Barzilai O (2022) Patient outcomes following implementation of an enhanced recovery after surgery pathway for patients with metastatic spine tumors. Cancer 128:4109–4118. https://doi.org/10.1002/cncr.34484
MacLean MA, Touchette CJ, Georgiopoulos M, Brunette-Clement T, Abduljabbar FH, Ames CP, Bettegowda C, Charest-Morin R, Dea N, Fehlings MG, Gokaslan ZL, Goodwin CR, Laufer I, Netzer C, Rhines LD, Sahgal A, Shin JH, Sciubba DM, Stephens BF, Fourney DR, Weber MH, Tumor AOSKF (2022) Systemic considerations for the surgical treatment of spinal metastatic disease: a scoping literature review. Lancet Oncol 23:e321–e333. https://doi.org/10.1016/S1470-2045(22)00126-7
Kanda Y, Kakutani K, Sakai Y, Yurube T, Miyazaki S, Takada T, Hoshino Y, Kuroda R (2020) Prospective cohort study of surgical outcome for spinal metastases in patients aged 70 years or older. Bone Joint J. 102:1709–1716. https://doi.org/10.1302/0301-620X.102B12.BJJ-2020-0566.R1
Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, Shepperd S (2016) Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010669.pub2
Jiang V, Brooks EM, Tong ST, Heintzman J, Krist AH (2020) Factors influencing uptake of changes to clinical preventive guidelines. J Am Board Fam Med 33:271–278. https://doi.org/10.3122/jabfm.2020.02.190146
Ryckman T, Weiser J, Gombe M, Turner K, Soni P, Tarlton D, Mazhidova N, Churchyard G, Chaisson RE, Dowdy DW (2023) Impact and cost-effectiveness of short-course tuberculosis preventive treatment for household contacts and people with HIV in 29 high-incidence countries: a modelling analysis. Lancet Glob Health 11:e1205–e1216. https://doi.org/10.1016/S2214-109X(23)00251-6
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L, Consortium ANS (2010) AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med 51:421–424. https://doi.org/10.1016/j.ypmed.2010.08.005
Qaseem A, Harrod CS, Crandall CJ, Wilt TJ, Clinical Guidelines Committee of the American College of P, Balk EM, Cooney TG, Cross JT, Jr., Fitterman N, Maroto M, Obley AJ, Tice J, Tufte JE, Shamliyan T, Yost J (2023) Screening for colorectal cancer in asymptomatic average-risk adults: a guidance statement from the American college of physicians (version 2). Ann Intern Med 176:1092–1100. https://doi.org/10.7326/M23-0779
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906. https://doi.org/10.1016/j.ijsu.2021.105906
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sadeghirad B, Johnston BC (2017) The scientific basis of guideline recommendations on sugar intake. Ann Intern Med 167:219. https://doi.org/10.7326/L17-0255
van Teijlingen E, Hundley V (2002) The importance of pilot studies. Nurs Stand 16:33–36. https://doi.org/10.7748/ns2002.06.16.40.33.c3214
Anderson DB, Luca K, Jensen RK, Eyles JP, Van Gelder JM, Friedly JL, Maher CG, Ferreira ML (2021) A critical appraisal of clinical practice guidelines for the treatment of lumbar spinal stenosis. Spine J 21:455–464. https://doi.org/10.1016/j.spinee.2020.10.022
Guan B, Li G, Zheng R, Fan Y, Yao L, Chen L, Feng S, Zhou H (2023) A critical appraisal of clinical practice guidelines for diagnostic imaging in the spinal cord injury. Spine J 23:1189–1198. https://doi.org/10.1016/j.spinee.2023.03.003
Zheng R, Guan B, Fan Y, Fu R, Yao L, Wang W, Li G, Chen L, Zhou H, Feng S (2023) A critical appraisal of clinical practice guidelines for management of four common complications after spinal cord injury. Spine J 23:888–899. https://doi.org/10.1016/j.spinee.2022.12.001
Erickson J, Sadeghirad B, Lytvyn L, Slavin J, Johnston BC (2017) The scientific basis of guideline recommendations on sugar intake: a systematic review. Ann Intern Med 166:257–267. https://doi.org/10.7326/M16-2020
Khanji MY, Bicalho VV, van Waardhuizen CN, Ferket BS, Petersen SE, Hunink MG (2016) Cardiovascular risk assessment: a systematic review of guidelines. Ann Intern Med 165:713–722. https://doi.org/10.7326/M16-1110
Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, Ueffing E, Andrews J, Alonso-Coello P, Meerpohl JJ, Lang DM, Jaeschke R, Williams JW, Jr., Phillips B, Lethaby A, Bossuyt P, Glasziou P, Helfand M, Watine J, Afilalo M, Welch V, Montedori A, Abraha I, Horvath AR, Bousquet J, Guyatt GH, Schunemann HJ, Group GW (2011) Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy 66:588–595. https://doi.org/10.1111/j.1398-9995.2010.02530.x
Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P, Helfand M, Ueffing E, Alonso-Coello P, Meerpohl J, Phillips B, Horvath AR, Bousquet J, Guyatt GH, Schunemann HJ, Group GW (2009) Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy 64:1109–1116. https://doi.org/10.1111/j.1398-9995.2009.02083.x
Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A, Bousquet J, Guyatt GH, Schunemann HJ, Group GW (2009) Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64:669–677. https://doi.org/10.1111/j.1398-9995.2009.01973.x
Expert Panel on Radiation Oncology-Bone M, Lo SS, Lutz ST, Chang EL, Galanopoulos N, Howell DD, Kim EY, Konski AA, Pandit-Taskar ND, Rose PS, Ryu S, Silverman LN, Sloan AE, Van Poznak C (2013) ACR appropriateness criteria (R) spinal bone metastases. J Palliat Med 16:9–19. https://doi.org/10.1089/jpm.2012.0376
Expert Panel on Radiation Oncology-Bone M, Lo SS, Ryu S, Chang EL, Galanopoulos N, Jones J, Kim EY, Kubicky CD, Lee CP, Rose PS, Sahgal A, Sloan AE, Teh BS, Traughber BJ, Van Poznak C, Vassil AD (2015) ACR appropriateness criteria(R) metastatic epidural spinal cord compression and recurrent spinal metastasis. J Palliat Med 18:573–584. https://doi.org/10.1089/jpm.2015.28999.sml
Bollen L, Dijkstra SPD, Bartels R, de Graeff A, Poelma DLH, Brouwer T, Algra PR, Kuijlen JMA, Minnema MC, Nijboer C, Rolf C, Sluis T, Terheggen M, van der Togt-van Leeuwen ACM, van der Linden YM, Taal W (2018) Clinical management of spinal metastases: the Dutch national guideline. Eur J Cancer 104:81–90. https://doi.org/10.1016/j.ejca.2018.08.028
Groenen KHJ, van der Linden YM, Brouwer T, Dijkstra SPD, de Graeff A, Algra PR, Kuijlen JMA, Minnema MC, Nijboer C, Poelma DLH, Rolf C, Sluis T, Terheggen M, van der Togt-van Leeuwen ACM, Bartels R, Taal W (2018) The Dutch national guideline on metastases and hematological malignancies localized within the spine; a multidisciplinary collaboration towards timely and proactive management. Cancer Treat Rev 69:29–38. https://doi.org/10.1016/j.ctrv.2018.05.013
Spratt DE, Beeler WH, de Moraes FY, Rhines LD, Gemmete JJ, Chaudhary N, Shultz DB, Smith SR, Berlin A, Dahele M, Slotman BJ, Younge KC, Bilsky M, Park P, Szerlip NJ (2017) An integrated multidisciplinary algorithm for the management of spinal metastases: an International spine oncology consortium report. Lancet Oncol 18:e720–e730. https://doi.org/10.1016/S1470-2045(17)30612-5
Altaf F, Weber M, Dea N, Boriani S, Ames C, Williams R, Verlaan JJ, Laufer I, Fisher CG (2016) Evidence-based review and survey of expert opinion of reconstruction of metastatic spine tumors. Spine (Phila Pa 1976) 41(Suppl 20):S254–S261. https://doi.org/10.1097/BRS.0000000000001819
(2019). In: 2019 surveillance of metastatic spinal cord compression in adults: risk assessment, diagnosis and management (NICE guideline CG75). London.
Gasbarrini A, Boriani S, Capanna R, Casadei R, Di Martino A, Silvia Spinelli M, Papapietro N, Piccioli A, Italian Orthopaedic Society Bone Metastasis Study G (2014) Management of patients with metastasis to the vertebrae: recommendations from the Italian orthopaedic society (SIOT) bone metastasis study group. Expert Rev Anticancer Ther 14:143–150. https://doi.org/10.1586/14737140.2014.856532
Choi SH, Koo JW, Choe D, Kang CN (2020) The incidence and management trends of metastatic spinal tumors in South Korea: a nationwide population-based study. Spine (Phila Pa 1976) 45:E856–E863. https://doi.org/10.1097/BRS.0000000000003445
Orenday-Barraza JM, Cavagnaro MJ, Avila MJ, Strouse IM, Dowell A, Kisana H, Khan N, Ravinsky R, Baaj AA (2022) 10-Year trends in the surgical management of patients with spinal metastases: a scoping review. World Neurosurg 157(170–186):e173. https://doi.org/10.1016/j.wneu.2021.10.086
Kumar N, Malhotra R, Zaw AS, Maharajan K, Naresh N, Kumar A, Vellayappan B (2017) Evolution in treatment strategy for metastatic spine disease: presently evolving modalities. Eur J Surg Oncol 43:1784–1801. https://doi.org/10.1016/j.ejso.2017.05.006
Gazzeri R, Telera S, Galarza M, Callovini GM, Isabella S, Alfieri A (2021) Surgical treatment of intramedullary spinal cord metastases: functional outcome and complications-a multicenter study. Neurosurg Rev 44:3267–3275. https://doi.org/10.1007/s10143-021-01491-8
Al Farii H, Aoude A, Al Shammasi A, Reynolds J, Weber M (2023) Surgical management of the metastatic spine disease: a review of the literature and proposed algorithm. Global Spine J 13:486–498. https://doi.org/10.1177/21925682221146741
Hendel RC, Lindsay BD, Allen JM, Brindis RG, Patel MR, White L, Winchester DE, Wolk MJ (2018) ACC appropriate use criteria methodology: 2018 update: a report of the American college of cardiology appropriate use criteria task force. J Am Coll Cardiol 71:935–948. https://doi.org/10.1016/j.jacc.2018.01.007
Tabourel G, Terrier LM, Dubory A, Cristini J, Nail LL, Cook AR, Buffenoir K, Pascal-Moussellard H, Carpentier A, Mathon B, Amelot A (2021) Are spine metastasis survival scoring systems outdated and do they underestimate life expectancy? Caution in surgical recommendation guidance. J Neurosurg Spine 35:527–534. https://doi.org/10.3171/2020.12.SPINE201741
Hu X, Huang W, Sun Z, Ye H, Man K, Wang Q, Sun Y, Yan W (2022) Predictive factors, preventive implications, and personalized surgical strategies for bone metastasis from lung cancer: population-based approach with a comprehensive cancer center-based study. EPMA J 13:57–75. https://doi.org/10.1007/s13167-022-00270-9
Liu Y, Cao X, Zhao X, Shi X, Lei M, Qin H (2022) Quality of life and mental health status among cancer patients with metastatic spinal disease. Front Public Health 10:916004. https://doi.org/10.3389/fpubh.2022.916004
Richardson MA, Bernstein DN, Kulp A, Mesfin A (2022) Patient Reported Outcomes in Metastatic Spine Disease: Concurrent Validity of PROMIS with the Spine Oncology Study Group Outcome Questionnaire. Spine (Phila Pa 1976) 47:591–596. doi: https://doi.org/10.1097/BRS.0000000000004327
Kato S, Demura S, Shinmura K, Yokogawa N, Shimizu T, Murakami H, Kawahara N, Tomita K, Tsuchiya H (2021) Surgical Metastasectomy in the Spine: A Review Article. Oncologist 26:e1833–e1843. https://doi.org/10.1002/onco.13840
Melcher I, Disch AC, Khodadadyan-Klostermann C, Tohtz S, Smolny M, Stockle U, Haas NP, Schaser KD (2007) Primary malignant bone tumors and solitary metastases of the thoracolumbar spine: results by management with total en bloc spondylectomy. Eur Spine J 16:1193–1202. https://doi.org/10.1007/s00586-006-0295-5
Funding
The study was supported by the Project of Shanghai Municipal Health Commission (20204Y0165, 20224Y0165), National Natural Science Foundation of China (82205145), and Shanghai “Rising Stars of Medical Talents”-Youth Development Program-Youth Medical Talents-Specialist Program SHWSRS (2023-062) and the Project of Chinese Society of Traditional Chinese Medicine youth talent lifting (2023-QNRC2-A03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1. Search strategies of all databases
PubMed
#1 Spinal Neoplasms"[Mesh].
#2 Spinal neoplasms"[Title/Abstract].
#3 spinal metastases"[Title/Abstract].
#4 spinal metastasis"[Title/Abstract].
#5 Spine metastases"[Title/Abstract].
#6 Vertebral metastases [Title/Abstract].
#7 Vertebral metastasis"[Title/Abstract].
#8 spine tumor"[Title/Abstract].
#9 Spinal Cord Neoplasm [Mesh].
#10 Spinal Cord Tumor"[Title/Abstract].
#11 Spinal Cord Neoplasm"[Title/Abstract].
#12 Spinal Cord Neoplasms"[Title/Abstract].
#13 Spinal Cord Tumors"[Title/Abstract].
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13.
#15 Guideline [Publication Type].
#16 Guidelines as Topic [Mesh].
#17 Practice Guideline [Publication Type].
#18 Guidelines [Title/Abstract].
#19 Guideline [Title/Abstract].
#20 #15 or #16 or #17 or #18 or #19.
#21 #20 and #14.
Web of Science
#1 TS = Spinal neoplasms.
#2 TS = spinal metastases.
#3 TS = spinal metastasis.
#4 TS = Spine metastases.
#5 TS = spine metastasis.
#6 TS = Vertebral metastases.
#7 TS = Vertebral metastasis.
#8 TS = spine tumor.
#9 TS = Spinal Cord Tumor.
#10 TS = Spinal Cord Neoplasm.
#11 TS = Spinal Cord Neoplasms.
#12 TS = Spinal Cord Tumors.
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR
#14 TS = (“Guidelines” OR "Guideline”).
#15 #13 AND #14
Embase
1 spine tumor./exp.
2 Spinal neoplasms.ti,ab,kw.
3 spinal metastases.ti,ab,kw.
4 spinal metastasis.ti,ab,kw.
5 Spine metastases.ti,ab,kw.
6 spine metastasis.ti,ab,kw.
7 Vertebral metastases.ti,ab,kw.
8 Vertebral metastasis.ti,ab,kw.
9 spinal cord tumor./exp.
10 Spinal Cord Tumor.ti,ab,kw.
11 Spinal Cord Neoplasm.ti,ab,kw.
12 Spinal Cord Neoplasms.ti,ab,kw.
13 Spinal Cord Tumors.ti,ab,kw.
14 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
15 practice guideline./exp.
16 Guidelines.ti,ab,kw.
17 Guideline.ti,ab,kw.
18 OR 17
19 AND 18
Organization | Website | Strategy |
|---|---|---|
National Institute for Health and Care Excellence (NICE) | Click “NICE guidance” Filtered by title or keyword “spinal metastases” OR “spinal neoplasms” OR “spinal tumor” | |
Scottish Intercollegiate Guidelines Network (SIGN) | Scanned guidelines Filtered by subspecialty “Spine” | |
Guidelines International Network(GIN) | Click “International Guidelines Library” Keyword search “spinal metastases” OR “spinal neoplasms” OR “spinal tumor” | |
Agency for Healthcare Research and Quality (AHRQ) | Search all AHRQ sites Terms searched “spinal metastases” OR “spinal neoplasms” OR “spinal tumor” |
Appendix 2. Appraisal of Guidelines for Research and Evaluation, 2nd edition (AGREE II) instrument
Item | Description |
|---|---|
Domain 1. Scope and purpose | |
Item 1 | The overall objective(s) of the guideline is (are) specifically described |
Item 2 | The health question(s) covered by the guideline is (are) specifically described |
Item 3 | The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described |
Domain 2. Stakeholder involvement | |
Item 4 | The guideline development group includes individuals from all relevant professional groups |
Item 5 | The views and preferences of the target population (patients, public, etc.) have been sought |
Item 6 | The target users of the guideline are clearly defined |
Domain 3. Rigor of development | |
Item 7 | Systematic methods were used to search for evidence |
Item 8 | The criteria for selecting the evidence are clearly described |
Item 9 | The strengths and limitations of the body of evidence are clearly described |
Item 10 | The methods for formulating the recommendations are clearly described |
Item 11 | The health benefits, side effects, and risks have been considered in formulating the recommendations |
Item 12 | There is an explicit link between the recommendations and the supporting evidence |
Item 13 | The guideline has been externally reviewed by experts prior to its publication |
Item 14 | A procedure for updating the guideline is provided |
Domain 4. Clarity of presentation | |
Item 15 | The recommendations are specific and unambiguous |
Item 16 | The different options for management of the condition or health issue are clearly presented |
Item 17 | Key recommendations are easily identifiable |
Domain 5. Applicability | |
Item 18 | The guideline describes facilitators and barriers to its application |
Item 19 | The guideline provides advice and/or tools on how the recommendations can be put into practice |
Item 20 | The potential resource implications of applying the recommendations have been considered |
Item 21 | The guideline presents monitoring and/or auditing criteria |
Domain 6. Editorial independence | |
Item 22 | The views of the funding body have not influenced the content of the guideline |
Item 23 | Competing interests of guideline development group members have been recorded and addressed |
Appendix 3. Assessment scale on level of evidence
Assessment Scale | Definition |
|---|---|
Grade A | “High” / “There is good evidence to support the recommendation that the condition be specifically considered in a periodic health examination”/ “Consistent level 1 studies (i.e., systematic review of randomized control trials, or individual randomized control trials)” |
Grade B | “Moderate” / “There is fair evidence to support the recommendation that the condition be specifically considered in a periodic health examination” / “Consistent level 2 (i.e., systematic review of cohort studies, or individual cohort studies) or level 3 (i.e., systematic review of case–control studies, or individual case–control studies) studies or extrapolations from level 1 studies” |
Grade C | “Low” / “There is poor evidence regarding the inclusion of the condition in a periodic health examination, but recommendations may be made on other grounds” / “Level 4 studies (i.e., case-series, poor quality cohort and case–control studies) or extrapolations from level 2 or 3 studies” |
Grade D | “Very low” / “Level 5 studies (i.e., expert opinion without explicit critical appraisal) or troublingly inconsistent or inconclusive studies of any level” |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, W., Chen, D., Ding, X. et al. A critical appraisal of clinical practice guidelines on surgical treatments for spinal metastasis. Eur Spine J (2024). https://doi.org/10.1007/s00586-023-08127-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-023-08127-z