Abstract
Purpose
To investigate the clinical application value of the non-shared incentive diffusion imaging technique (ZOOM-DWI) diagnoses of cervical spondylotic myelopathy (CSM).
Methods
49 CSM patients who presented from January 2022 to December 2022 were selected as the patient group, and 50 healthy volunteers are recruited as the control group. All subjects underwent conventional MRI and ZOOM-DWI of the cervical spine and neurologic mJOA scores in patients with CSM. The spinal ADC values of segments C2-3, C4-5, C5-6, and C6-7 are measured and analyzed in all subjects, with C5-6 being the most severe level of spinal canal compression in the patient group. In addition, the study also analyzes and compares the relationship between the C5-6 ADC value and mJOA score in the patient group.
Results
The mean ADC shows no significantly different levels in the control group. Among the ADC values at each measurement level in the patient group, except for C4-5 and C6-7 segments are not statistically significant, the remaining pair-wise comparisons all show statistically significant differences (F = 24.368, p < 0.001). And these individuals have the highest ADC value at C5-6. The C5-6 ADC value in the patient group is significantly higher compared with the ADC value in the control group (t = 9.414, p < 0.001), with statistical significance. The ADC value at the patient stenosis shows a significant negative correlation with the mJOA score (r = −0.493, p < 0.001).
Conclusion
Cervical ZOOM-DWI can be applied to diagnose CSM, and spinal ADC value can use as reliable imaging data for diagnosing cervical myelopathy.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) is a common neurological disorder caused by degenerative changes in the spine that lead to compression of the cervical spinal cord. Conventional MRI technology is not sensitive to the early diagnosis of CSM and cannot accurately assess the neurological impairment of patients [1, 2]. In recent years, diffusion imaging technology has developed rapidly. At present, studies have pointed out that Diffusion tensor imaging (DTI) indicators, such as mean diffusivity (MD) value and fractional anisotropy (FA) value, can predict the postoperative recovery of CSM [3, 4]. However, due to the long imaging time of DTI, patients are not easy to tolerate, which is likely to lead to the limitation of clinical application.
ZOOM-DWI (Philips Healthcare, Netherlands), also known as non-public excitation diffusion imaging, is a small-field imaging technique. It combines an ordinary 90°radiofrequency pulse with an oblique 180°reunion pulse to select and excite a local field of view (FOV). With the application of a saturated band, it can achieve the purpose of reducing FOV while not producing curl, to obtain a high-quality image. Compared with ordinary DWI images, ZOOM-DWI technology has shorter imaging time, lower magnetic susceptibility artifact, and higher blood contrast, and its advantages have been confirmed by previous studies in the imaging of thyroid, pituitary, and other organs with a small field of vision [5].
In this study, the value of ZOOM-DWI in the diagnosis of cervical spondylotic myelopathy was diskussed by analyzing the changes in cervical spinal cord ADC values in patients with CSM.
Data and methods
General information
49 CSM patients who presented from January 2022 to December 2022 are selected as the patient group (mean age 52.6 ± 11.2 years old, 34 men and 15 women). The most severe vertebral canal compression segment in the patient group is C5-6. During the study, 50 healthy volunteers matched with the patients in gender and age selected as the control group (mean age 54.7 ± 9.0 years old, 34 men and 16 women). All volunteers in the patient group and control group underwent routine cervical vertebra and ZOOM-DWI examinations after admission.
Inclusion criteria: (1) Classic signs and symptoms of CSM; (2) There is no contraindication of MRI. (3) Able to cooperate with the inspector smoothly.
Exclusion criteria: (1) Patients with metal implants; (2) lactating and pregnant women; (3) Postoperative patients with cervical spine; (4) Spinal cord tumors, trauma, inflammation, and demyelinating diseases.
Neurological function assessment
Evaluation of spinal cord function using the mJOA score. This scale was revised by the Japanese Orthopedic Association on March 18, 1994, to evaluate spinal cord nerve function in patients with cervical spondylosis, with a total score of 17. Including upper limb motor function, lower limb motor function, upper limb and trunk sensory disorders, and bladder function. The neurological function is evaluated in patients with CSM before surgery. The mJOA score is performed by two experienced clinical attending physicians, and the average of the two physicians' evaluations is taken as the mJOA score.
Routine MRI and ZOOM-DWI scanning
Philips ingenia-cx model is used for conventional plain MRI and ZOOM-DWI examination of cervical vertebra in all volunteers. Conventional MRI scans include sagittal T2WI, sagittal T1WI, and the STIR sequence in the sagittal positions. The patients are placed in the supine position.
The sequence scanning parameters of the cervical spinal cord are as follows: Sagittal T1WI: TR 590 ms, TE 7.5 ms, FOV 160 mm × 229 mm; Sagittal T2WI: TR 2500 ms, TE 110 ms, FOV 161 mm × 232 mm; Sagittal STIR: TR 2500 ms, TE 60 ms, FOV 60 mm × 228 mm; ZOOM-DWI: FOV 220 × 60 mm, acquisition matrix 88 × 24, TR 3000 ms, TE 53 ms, scanning layer number 13, layer thickness 3 mm, NSA = 1. Two b values are used in the study, b = 0 s/mm2 and b = 400 s/mm2.
Image post-processing
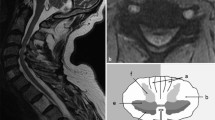
Raw images are sent to the Philips workstation: Nebula Workstation (IntelliSpace Portal) 9.0, Neuro Perfusion for processing. The narrowest part (C5-6) determines sagittal T2WI images of the cervical spine, and ADC values at all levels measures on sagittal images of ZOOM-DWI, with a region of interest (ROI) of about 10–15mm2, as shown in Fig. 1. The above data measure for three times in each case and averaged.
Statistical analysis
Statistical analysis is performed in the control group using EXCEL and SPSS 26.0 software, and all values are expressed as "mean ± standard deviation". Intragroup comparison of the spinal cord ADC values between the patient group and the control group is performed using variance analysis and SNK-q. T-test of independent samples is performed on the narrowest part of the patient group and ADC values of each measured segment and corresponding segment. Pearson correlation analysis is performed between ADC values at patients' stenosis and mJOA scores, and the receiver operator characteristic curve (ROC curve) is drawn for analysis. p < 0.05 means the difference is statistically significant.
Results
All the subjects in the patient group and the control group complete the routine sequence and the ZOOM-DWI sequence scanning, as shown in Figs. 2 and 3.
A male 58-year-old patient with csm. a sagittal plane T1WI; b sagittal T2WI (visible high signal in C5-6); c sagittal STIR lipogram (visible high signal in C5-6); d two-dimensional ZOOM-DWI color map (visible increased signal of corresponding position); e ZOOM-DWI (visible increased signal of corresponding position); f ADC diagram
Table 1 shows that there are no statistical differences between patients and controls; The four-square chi-square test shows that p = 0.882 > 0.05, indicating no statistical differences in gender between the two groups.
Comparison of ADC values in each segment of the control group
There are no significant differences in the values of C2-3 ADC (0.84 ± 0.16), C4-5 ADC (0.83 ± 0.16), C5-6 ADC (0.86 ± 0.17), and C6-7 ADC (0.85 ± 0.18) at different segments of the control group (F = 0.274, p = 0.824 > 0.05).
Comparison of ADC values in each segment of the CSM patient group
The value of C4-5 ADC (1.04 ± 0.26) is significantly higher than C2-3 ADC (F = 18.708, p < 0.001). The value of C5-6 ADC (1.22 ± 0.20) is significantly higher than C2-3 ADC (0.85 ± 0.18) (F = 90.021, p < 0.001). The value of C6-7 ADC (1.06 ± 0.21) is significantly higher than that of C2-3 ADC (0.85 ± 0.18) (F = 28.347, p < 0.001). The value of C5-6 ADC (1.22 ± 0.20) is significantly higher than C4-5 ADC (1.04 ± 0.26) (F = 13.698, p < 0.001). The ADC value of C5-6 (1.22 ± 0.20) is significantly higher than that of C6-7 (1.06 ± 0.21) (F = 14.889, p < 0.001), and the differences are statistically significant. There are no significant differences between C6-7 ADC (1.06 ± 0.21) and C4-5 ADC (1.04 ± 0.26) (F = 0.09, p = 0.744 > 0.05).
Comparison of ADC values in each segment between CSM patients and the control group
The value of ADC at C5-6 in the narrowest part of the patient group is significantly higher than that in the control group (t = 9.414, p < 0.001). ADC values at C4-5 in the upper segment are significantly higher than the control group (t = 4.927, p < 0.001). ADC values at C6-7 in the next stage are significantly higher than those in the control group (t = 5.284, p < 0.001). The above differences are statistically significant. However, there are no significant differences in ADC value at C2-3 between the patient group and the control group (t = 0.152, p = 0.880 > 0.05). See Table 2 for details.
Correlation analysis between ADC value at the narrowest point and mJOA score
The preoperative mJOA score of 49 patients with CSM is (11.31 ± 2.78). Pearson correlation analysis is conducted between ADC values at the narrowest segment of patients and their preoperative mJOA scores. See Fig. 4 for the scatter plot and fitting equation and Fig. 5 for the ROC curve. The results show that the ADC value at the most obvious spinal canal compression is significantly negatively correlated with the mJOA score (r = −0.493, p < 0.001). The area under the ROC curve AUC = 0.907 > 0.5, and the cut-off value is 0.99.
Discussion
The imaging techniques used in this study are different from those used in previous studies, but both explore the role of ADC values in disease diagnosis. Diffusion tensor imaging (DTI) quantitative indicators, such as ADC value, FA value, MD value, etc. According to the research of Schöller K [3], Zheng, WP [4], Banaszek A [6], and Wang K [7], some quantitative indicators of DTI can also be used as the diagnostic basis for CSM. As one of the important components of fMRI, DTI can quantitatively analyze the diffusion movement of water molecules in tissues in three-dimensional space compared with DWI. Due to the anisotropic characteristics of water diffusion in tissues, it can show different tissue structures, which is of great significance in the study of white matter fiber bundles in the brain [8, 9]. FA value and MD value, as two quantitative indexes of DTI, have been used many times in previous studies. The difference between the two is that FA value reflects the anisotropic diffusion degree of water molecules in white matter fibers, and mainly explores the directivity and integrity of fiber bundles. The MD value reflects the speed of diffusion movement and mainly explores the integrity and size of tissue cells. However, the imaging time of DTI is long, which is difficult to meet clinical requirements. Therefore, DWI with more time advantage is considered in this experiment. In this paper, whether ZOOM-DWI technology can be applied to the diagnosis of clinical CSM is diskussed. Compared with traditional DWI reflects tissue microstructural changes, ZOOM-DWI has greater advantages and potential due to shorter imaging time, lower susceptibility artifacts, and higher blood contrast. During the experiment, ZOOM-DWI technology is used to measure the change of ADC value and detect the diffusion changes of water molecules in the spinal cord, which could reflect the subtle cervical spinal cord injury and identify early myelopathy, which was more sensitive than conventional cervical MRI [10].
The comparison between the patient group and the control group showed that: First, ADC values of different segments in the control group are not different. It can be proved that the experimental method is consistent and reproducible. Second, there are no differences in the ADC values of the upper and lower segments of the narrowest segment in the CSM patient group. The closer the segment to the most obvious stenosis, the higher the ADC value, while the farther the segment from the narrowest has little influence on the ADC values. As can be seen from the above, compared with the normal population without CSM, ADC values at and near this segment will also increase with the increase in the severity of cervical spinal cord compression in patients with CSM. In ZOOM-DWI technology, ADC value serves as its basic quantitative index, which can be used to describe the velocity and range of diffusion motion of water molecules in different directions in the organizational structure of the DWI sequence. The larger the ADC value is, the stronger the dispersion motion of water molecules is [11]. Since early axon damage in CSM patients will change the direction of water molecules, ZOOM-DWI can provide ideas for the early detection of CSM \* MERGEFORMAT [12].
The results of each segment comparison between the patient group and the control group show that compared with the normal cervical spinal cord structure, ADC value increases with the increasing severity of the cervical spinal cord compression site. Starting from the pathophysiological mechanism of the disease, CSM often begins with degeneration and collapse of the intervertebral disk, followed by a narrowing of intervertebral space, and then loss of normal cervical lordosis. During the progression of cervical degeneration, neurological structures will also be damaged. ADC value can be quantified as an indicator of the diffusion intensity of water molecules in human tissues. When an axon is damaged, the movement of water molecules will not be restricted, increasing ADC value [13,14,15]. Similarly, the larger the ADC value, the stronger the molecular diffusion ability of water can be quantified, which indicates the destruction of the fiber cell membrane or myelin sheath \* MERGEFORMAT [16, 17]. Therefore, ADC value can provide ideas for the early detection of CSM. According to the experimental results, the ADC value of the cervical spinal cord just reflects the severity of the disease, and correlation analysis also supports this view.
Correlation analysis results show that the higher the ADC value in the narrowest part of CSM patients, the lower the clinical mJOA score of corresponding patients, that is, the more serious the disease. It can be concluded that ADC value is significantly correlated with the severity of cervical spinal cord compression in CSM, which is expected to be applied in the clinic. Spinal cord injury caused by mechanical compression, abnormal intersegmental activity caused by instability of the cervical spine, and disorders of spinal blood circulation is often considered the pathogenesis of cervical spondylotic myelopathy \* MERGEFORMAT [18]. Sato [19] shows that ADC value is closely related to the severity of CSM symptoms. Because the compression may cause damage to the nerve structure, which makes the activity of water molecules in the cervical spinal cord unrestricted, the higher the ADC value, the more severe the patient's symptoms will be. This also explains why the ADC value of C5-6, the segment with the most severe cervical spinal cord compression in the CSM group, is the highest, and the upper and lower segments show significant statistical differences compared with the control group, which is also consistent with the previous experimental results. The ROC curve is also drawn in this paper to directly reflect the sensitivity and specificity of this diagnostic method. The larger the AUC value under the ROC curve, the better the classification effect. Therefore, ADC value can provide a new imaging parameter for the diagnosis and treatment of clinical CSM.
The experiment has its limitations and shortcomings. First, in terms of sample selection: 49 CSM patients and 50 healthy volunteers are randomly selected in the experiment, a total of 99 people. Although the selected samples could prove the corresponding statistical relationship, if more sufficient samples could be found, the results would be more accurate. Second, in terms of data acquisition: the CSM patients selected in this experiment all have the most severe cervical spinal cord compression at C5-6 segments, and the compression level of patients is single. Although the experimental results are valid, follow-up verification should conduct for CSM patients with compression at other segments.
To sum up, the ZOOM-DWI technology mentioned in this paper objectively and quantitatively describes the feasibility of cervical spinal cord ADC value in the clinical diagnosis of cervical spondylotic myelopathy. Cervical ZOOM-DWI can be applied in the diagnosis of CSM, and spinal cord ADC value can be used as reliable imaging data for the diagnosis of cervical spondylotic myelopathy.
References
Okita G, Ohba T, Takamura T et al (2018) Application of neurite orientation dispersion and density imaging or diffusion tensor imaging to quantify the severity of cervical spondylotic myelopathy and to assess postoperative neurologic recovery. Spine J 18(2):268–275. https://doi.org/10.1016/j.spinee.2017.07.007
Hori M, Fukunaga I, Masutani Y et al (2012) New diffusion metrics for spondylotic myelopathy at an early clinical stage [J]. Eur Radiol 22(8):1797–1802. https://doi.org/10.1007/s00330-012-2410-9
Schöller K, Siller S, Brem C et al (2020) Diffusion tensor imaging for surgical planning in patients with cervical spondylotic myelopathy. J Neurol Surg Part A. Central European neurosurgery. 81(A1):1–9. https://doi.org/10.1055/s-0039-1691822
Zheng W, Chen H, Wang N et al (2018) Application of diffusion tensor imaging cutoff value to evaluate the severity and postoperative neurologic recovery of cervical spondylotic myelopathy. World Neurosurg 118:e849–e855. https://doi.org/10.1016/j.wneu.2018.07.067
Liu R, Jiang G, Gao P et al (2018) Non-invasive amide proton transfer imaging and zoom diffusion-weighted imaging in differentiating benign and malignant thyroid micronodules. Front Endocrinol (Lausanne 9:747. https://doi.org/10.3389/fendo.2018.00747
Banaszek A, Bladowska J, Podgórski P, Sąsiadek MJ (2016) Role of diffusion tensor MR imaging in degenerative cervical spine disease: a review of the literature. Clin Neuroradiol 26(3):265–276. https://doi.org/10.1007/s00062-015-0467-y
Wang K, Chen Z, Zhang F et al (2017) Evaluation of DTI parameter ratios and diffusion tensor tractography grading in the diagnosis and prognosis prediction of cervical spondylotic myelopathy. Spine 42(4):E202–E210. https://doi.org/10.1097/BRS.0000000000001784
McLachlin S, Leung J, Sivan V et al (2021) Spatial correspondence of spinal cord white matter tracts using diffusion tensor imaging, fiber tractography, and atlas-based segmentation. Neuroradiology 63(3):373–380. https://doi.org/10.1007/s00234-021-02635-9
Jang SH, Seo JP (2021) Anatomical location of the spinothalamic tract in the subcortical white matter in the human brain: a diffusion tensor imaging study. Clin Anat 34(5):736–741. https://doi.org/10.1002/ca.23709
Ulubaba HE, Saglik S, Yildirim IO, Durak MA (2021) Effectiveness of diffusion tensor imaging in determining cervical spondylotic myelopathy. Turk Neurosurg 31(1):67–72. https://doi.org/10.5137/1019-5149.JTN.29149-20.2
Xu X, Zhang B, Sun K et al (2020) Reliability and validity of multi-shot DWI in diagnosis of cervical spondylotic myelopathy: a study based on 3-T MRI. Eur Spine J 29:1219–1226. https://doi.org/10.1007/s00586-020-06381-z
Edlow BL, Giacino JT, Hirschberg RE et al (2013) Unexpected recovery of function after severe traumatic brain injury: the limits of early neuroimaging-based outcome prediction. Neurocrit Care 19(3):364–375. https://doi.org/10.1007/s12028-013-9870-x
Nukala M, Abraham J, Khandige G et al (2018) Efficacy of diffusion tensor imaging in identification of degenerative cervical spondylotic myelopathy. Eur J Radiol Open. 6:16–23. https://doi.org/10.1016/j.ejro.2018.08.006
Rajasekaran S, Yerramshetty JS, Chittode VS et al (2014) The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine 39:1183–1189. https://doi.org/10.1097/BRS.0000000000000369
Tian X, Zhang L, Zhang X et al (2021) Correlations between preoperative diffusion tensor imaging and surgical outcome in patients with cervical spondylotic myelopathy. Am J Transl Res. 13(10):11461–11471. PMID: 34786072; PMCID: PMC8581918.
Basser PJ, Mattiello J, Lebihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66(1):259–267. https://doi.org/10.1016/S0006-3495(94)80775-1
Pierpaoli C, Jezzard P, Basser PJ et al (1996) Diffusion tensor MR imaging of the human brain. Radiology 201(3):637–648
Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. Spine J 6(6 Suppl):190S-197S. https://doi.org/10.1016/j.spinee.2006.04.024
Sato T, Horikoshi T, Watanabe A et al (2012) Evaluation of cervical myelopathy using apparent diffusion coefficient measured by diffusion-weighted imaging. AJNR Am J Neuroradiol 33(2):388–392. https://doi.org/10.3174/ajnr.A2756
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Tian, XN., Zhao, BG. et al. Diagnostic value of cervical spine ZOOM-DWI in cervical spondylotic myelopathy. Eur Spine J 33, 1223–1229 (2024). https://doi.org/10.1007/s00586-023-08110-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-08110-8