Abstract
Objective
Conventional freehand methods of pedicle screw placement are associated with significant complications due to close proximity to neural and vascular structures. Recent advances in augmented reality surgical navigation (ARSN) have led to its adoption into spine surgery. However, little is known regarding its overall accuracy. The purpose of this study is to delineate the overall accuracy of ARSN pedicle screw placement across various models.
Methods
A systematic review was conducted of Medline/PubMed, Cochrane and Embase Library databases according to the PRISMA guidelines. Relevant data extracted included reports of pedicle screw placement accuracy and breaches, as defined by the Gertzbein–Robbins classification, in addition to deviation from pre-planned trajectory and entry point. Accuracy was defined as the summation of grade 0 and grade 1 events per the Gertzbein–Robbins classification.
Results
Twenty studies reported clinically accurate placed screws. The range of clinically accurate placed screws was 26.3–100%, with 2095 screws (93.1%) being deemed clinically accurate. Furthermore, 5.4% (112/2088) of screws were reported as grade two breaches, 1.6% (33/2088) grade 3 breaches, 3.1% (29/926) medial breaches and 2.3% (21/926) lateral breaches. Mean linear deviation ranged from 1.3 to 5.99 mm, while mean angular/trajectory deviation ranged 1.6°–5.88°.
Conclusion
The results of this study highlight the overall accuracy of ARSN pedicle screw placement. However, further robust prospective studies are needed to accurately compare to conventional methods of pedicle screw placement.
Similar content being viewed by others
Introduction
Due to the proximity of pivotal neural and vascular structures, pedicle screw placement can be associated with significant complication rates [1, 2]. To ensure safe pedicle screw placement, assistive methods such as utilizing anatomic landmarks and fluoroscopic or computed tomography-guided imaging have been employed, with exposure to notable radiation for both the patient and theatre staff in the process. Other methods (e.g. mechanical drilling, 3D-printed preoperative planning) have been implemented in an attempt to reduce radiation exposure [2, 3]. However, these methods can prove expensive and prolong preoperative planning. Additionally, rates of accurate pedicle screw placement still vary considerably, thus questioning their overall efficacy.
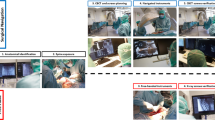
More recently, there has been notable excitement around developments in the realm of augmented reality and potential applications for the field of spine surgery. Augmented reality intraoperative navigation (ARSN) improves visualization of the surgical field with 3D intraoperative imaging produced via optical cameras incorporated into a ceiling-mounted C-arm (Fig. 1), aided by adhesive skin markers that facilitate tracking of patient position [3,4,5]. This allows surgeons to view an ideal trajectory path for pedicle screw placement superimposed on subjective visual fields through a headset, while simultaneously viewing the surgical field [1,2,3]. Furthermore, there is no need for pre-registration due to an integrated system. A recent systematic review by Vavra et al. [1] reports an accuracy range of 1–5 mm for augmented reality systems, highlighting its safety and efficacy across a variety of surgical procedures. However, little is known regarding the collective efficacy of ARSN for pedicle screw placement in spine surgery.
A and B: augmented reality with optical camera-based navigation and tracking highlighting the trajectory path from an oblique view at different time points, adapted from Elmi-Terander et al. [5] (2016) under the creative commons attribution-non-commercial license 4.0 (CCBY-NC), where it is permissible to download, share, remix, transform and build-up the work provided it is properly cited and not used for commercial purposes
Methods
Two independent reviewers (J.M.M and S.Y) performed a literature search per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6]. In the case of disagreement, a senior author was consulted (J.S.B). A comprehensive search was performed for eligible articles using the Medline/PubMed, Cochrane and Embase database to include studies up to, and including July 1st 2022. Search terms included “augmented reality” and “spine surgery” and “pedicle screw placement”. Inclusion criteria were predefined as comparative (i) clinical or cadaveric comparative studies, (ii) non-comparative studies and (iii) phantom or printed models. Exclusion criteria were articles not available in English. Bibliographies of retrieved, full-text articles were screened for further studies meeting the inclusion criteria.
Relevant data extracted were results of screw placement accuracy and breaches, as defined by the Gertzbein–Robbins classification; grade 0 (screw within the pedicle without cortical breach), grade 1 (0–2 mm breach, minor perforation including cortical encroachment), grade 2 (2–4 mm breach, moderate breach), grade 3 (more than 4 mm breach, severe displacement), grade 4 (4–6 mm breach) and grade 5 (> 6 mm breach). Accuracy was defined as the summation of grade 0 and grade 1 events per the Gertzbein–Robbins classification [7]. Additionally, events of lateral and medial breaches and mean deviation from preoperative plans were also collected. All extracted data was collated and compared for augmented reality-assisted pedicle screw placement.
Results
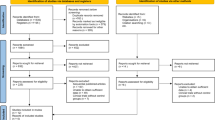
The initial literature search produced 104 results. After the removal of duplicates, 93 remained. Seventy-two articles were excluded after a review of the title and abstract. Thus, 27 studies were included for full-text review, with 23 studies subject to qualitative synthesis (Fig. 2).
There was a total of 74 cadavers (including 2 pig cadavers), 118 patients, 61 phantom models and 80 sawbone (agar-based) models across all studies. In terms of study design, 14 studies were cadaver, four mixed methods clinical approach, three phantom, one sawbone models in agar-based gel and one prospective case series. Overall, 2232 ARSN screws were placed. The basic characteristics of each study are outlined in Table 1. Of note, one study was omitted from further analysis in terms of collectively evaluating overall accuracy and respective breaches as the ARSN patient cohort was included in an additional study [18, 26]. Thus, only one of the two studies was included for analysis.
Clinically accurate placement
Twenty studies reported clinically accurate placed screws (Gertzbein and Robbins Grade 0+1). The range of ARSN clinically accurate placed screws was 26.3–100%. Out of the 2232 total ARSN screws that were placed, 2095 (93.1%) were deemed clinically accurate (Table 2). Comparatively, cadaver (1155/1237; 93.4%) and clinical studies (777/827; 93.4%) showed improved accuracy over phantom/sawbones models (123/168; 73.2%).
Grade 0
Seventeen studies reported grade 0 breaches. nine studies were cadaver, four were clinical and one was phantom. Grade 0 breaches ranged from 0 to 100%. A total of 2088 screws were placed, of which 1395 were reported as grade 0 breaches (66.8%) (Table 3).
Grade 1 breaches
Seventeen studies indicated grade 1 breaches. The range of grade 1 breaches was 0.0–95.6%. Overall, there were 595/2088 (28.5%) grade 1 breaches out of all screws placed (Table 4).
Grade 2 breaches
Grade 2 breaches were described in seventeen studies. Grade 2 breaches ranged from 0.0–45%, with a collective 5.4% of screws placed (112/2088) representing grade 2 breaches (Table 5).
Grade 3 breaches
Similarly, grade 3 breaches were reported in seventeen studies. Three, with a range of 0.0–6.3%. A total of 2088 were placed, of which 33 (1.6%) were grade 3 breaches (Table 6).
Grades 4 and 5 breaches
Only two studies reported grades 4 and 5 breaches [11, 19]. Molina et al. [19] reported one grade 4 (1/120; 0.8%) and three grade 5 breaches (3/120; 2.5%), while Farshad et al. [11] reported one (1/80; 1.3%) grade 4 breach.
Medial breaches
Nine studies reported medial breaches, with a range of 0.0–52.6% of screws placed. From the 926 screws placed, there were 29 medial breaches (3.1%), as outlined in Table 7.
Lateral breaches
Nine studies included lateral breaches. Incidence ranged from 0.0 to 20.8% across respective studies, which represented 2.3% (21/926) of screws placed (Table 8).
Mean linear and mean trajectory/angular deviation
Mean linear deviation was described in four studies, with a range of 1.3–5.99 mm. Mean angular/trajectory deviation was reported in six studies. The range of deviation across studies was 1.6°–5.88°. Results of individual studies are outlined in Table 9.
Discussion
Since the inception of virtual reality by Robert Mann in 1965, augmented reality has been adapted across various sectors and recently, healthcare. To date, studies depict the successful application of AR navigation systems across several surgical fields [27], with neurosurgery particularly innovative regarding the early adoption of augmented reality into clinical practice. For example, Skyrman et al. [28] demonstrated that the mean accuracy for percutaneous cranial biopsy needle insertions (n = 30) was 0.8 mm ± 0.43 mm, deemed an acceptable discrepancy from pre-planned trajectories. Demonstrated accuracy is further complimented by its efficiency in terms of workflow, with implementation of AR resulting in similar operative times to that of conventional methods [28]. Furthermore, the versatility of AR application is depicted in the literature as with Cercenelli et al. [29] who report a comparable success rate (100% vs 97%) in task-related osteo-myocutaneous fibular flap reconstructive surgery on 3D-printed leg phantoms (HoloLens vs tablet), with the AR model showing potential to minimize registration error in clinical practice [29].
In terms of orthopaedics, the first VR orthopaedic application was a knee arthroscopy simulator introduced in the 1990s. Since then, AR has been employed in numerous educational settings [30], in addition to pre-clinical diagnostic and therapeutic procedures [31, 32]. Tsukada et al. [33] demonstrated in a sawbones model that an AR-KNEE system was efficacious as CT measurements for global alignment in total knee arthroplasty (TKA) models. The absolute differences between the values displayed on the smartphone screen and CT measurement values for varus/valgus, posterior slope, internal/external rotation angles and thickness of the resected bone were 0.5° ± 0.2° (less than 1°), 0.8° ± 0.9° (less than 1°), 1.8° ± 1.5° (< 2°) and 0.6 mm ± 0.7 mm, respectively. Thus, this study highlights the reliable and reproducible accuracy of the AR system for coronal, sagittal and rotational alignment in tibial bone resection during TKA [33]. Additionally, Cho et al. [34] demonstrated in a porcine femur model of template introduced tumours, that the AR system demonstrated improved accuracy (p < 0.05) of resection compared to that of conventional resection. One hundred and sixty-four tumours were resected in 82 femurs in the AR group, with a mean error of 1.71 mm (range 0–6 mm). Comparably, 82 tumours were resected in 41 femurs in the conventional resection group, with a mean error of 2.64 mm (range 0–11 mm), with the AR group more probable to attain a 10 mm surgical margin compared to the conventional group (90.2% vs 70.7%) [34].
In spine surgery, AR has been used to assist pedicle screw insertion in phantom, pre-clinical and recently clinical models. ARSN improves visualization of the surgical field with 3D intraoperative imaging produced via optical cameras incorporated into a ceiling-mounted C-arm, aided by adhesive skin markers that facilitate tracking of patient position. A video-based system providing augmented reality surgical navigation ARSN with intraoperative 3D imaging proves the latest advancement in computer-assisted navigation. Furthermore, it has been shown to be accurate, safe and effective for pedicle screw placement [26]. Nevertheless, it remains in its infancy, with only five clinical studies and seven comparative studies to conventional techniques noted in the literature to date. Peh et al. [21] report in a cadaveric model that AR placed lumbar pedicle screws showed an improved comparative accuracy to that of fluoroscopy-guided screw placement (64/68; 94.2% vs 60/68; 88.2%), with reproducible accuracy across studies in both open and percutaneous/minimally invasive approaches. Pre-clinical findings are corroborated by recent clinical studies as demonstrated by Gu et al. 2020 [23], who in a mixed methods study of 50 patients undergoing lumbar pedicle screw placement, show improved accuracy for AR-guided screws (136/142; 95.77%) compared to conventional freehand technique (123/138; 89.13%). In addition to accuracy and avoidance of breaching the anterior cortex of vertebral body, the importance on accuracy in the axial plane and mitigation of lateral and medical breaches is integral. Three studies (all pre-clinical) report on lateral breaches for AR vs conventional technique; Elmi-Terander et al. [26] (5/23; 21.7% vs 15/33; 45.5%), Peh [21] (2/29; 6.7% vs 7/21; 33.3%), Urakov [15] (3/18; 16.7% vs 3/10; 30%), all showing reduced rates of lateral breaches for AR technology. In contrast, the same studies higher rates of medial breaches with ARSN; Elmi-Terander et al. [26] (2/23; 8.7% vs 2/33; 6.1%), Peh [21] (2/29; 6.7% vs 1/21; 4.7%), Urakov [25] (10/18; 55.5% vs 3/10; 30%). Such findings highlight certain obstacles to further clinical implementation of AR technology of thoracolumbar screws, particularly if there are reduced occurrences of medial breaches, due to concerns of the narrow spinal canal in the thoracic region. However, a reduced degree of lateral breaches could provide encouragement to the novel application for AR-assisted pedicle screw placement in the cervical region, and mitigate concerns due to the close proximity of vertebral arteries to cervical pedicles, with similar accuracy rates to novel intraoperative navigation systems employed for similar purposes [35, 36]. However, ARSN has certain advantages such as absence of intraoperative radiation experienced with intraoperative C-arm, and a reference field framed around the surgical site rather than one particular point of reference that may need to located a number of vertebrae away from the vertebrae level of concern. This can prove vital as shown that the further the distance from the vertebrae level of interest that the reference is located, the greater potential for inaccuracy [36].
Nevertheless, there are some obstacles to the widespread clinical adoption of AR-assisted spine surgery, in addition to the one aforementioned. It is currently unclear the degree of the learning curve associated with AR technology. The learning curve is a commonly used term in surgery to describe the process of demonstrating proficiency in a consistent manner. Several ARSN models exist on the market, whose comparative characteristics are not within the scope of this article. None of the studies included in this study report on the associated learning curve with AR-assisted pedicle screw placement. Furthermore, many of the studies are inclusive of experienced surgeons. As such, formal studies are needed to conduct and evaluate the learning curve for AR-assisted pedicle screw placement across varied levels of experience, with formal assessment measures such as objective structured assessment of surgical skills (OSATS) [36]. Secondly, institutions must aware of their capacity to afford the initial capital costs associated with erecting an AR-assisted spine surgery program. However, it is often considered a low-cost alternative requiring minimum infrastructure compared to certain intraoperative navigation systems and can be employed as a supplement to existing equipment for procedures other than pedicle screw placement, such as operating microscopes [37]. Other limitations include micromovement of fiducial markers which can affect accuracy, inadequate brightness or inattention blindness from natural light and battery life of AR head-packs [38, 39]. However, this is expected to be addressed and rectified as models advance, and AR may serve a cost effective alternative to navigation or robotic systems for smaller institutions wishing to provide modern surgical techniques for improved radiological and clinical outcomes. The mitigation of such concerns will further compliment technical advantages such as negating the need for surgeons to switch their eyeline from a 3D surgical field to a 2D bedside navigation system, and the absence of a reference frame in the surgical field, a current feature of certain navigation systems.
Computerised spine navigation using robotics and augmented reality is becoming more frequently used and is appealing to surgeons for both open and minimally invasive procedures. Comparing accuracy of different methods of pedicle screw placement yields interesting results [41]. Fatima et al. examined the differences of pedicle screw placement between robotic-assisted placement using augmented reality and freehand surgery. They found that patients undergoing robot-assisted screw placement had 1.68-fold greater likelihood of achieving ‘perfect’ accuracy, defined as when the screws were completely situated within the pedicle [41]. The meta-analysis by Fatima et al. also compared robotic-guided and fluoro-guided instrumentation placement in minimally invasive surgery. It found that there was a significantly higher risk of complications among patients with fluoro-guided surgery (OR 12.2; 95% CI 4.1–36.3) [41]. Importantly, this study declined to conclude that robotic guidance is superior to freehand techniques based on the quality of the data [41]. Comparing pedicle screw placement accuracy between freehand and computer-aided placement using augmented reality, Amiot et al. found that in screws placed between T5 and S1, freehand placement had an error rate of 15.3% from 544 cases, whereas there was a 5.4% error rate from 294 cases when computer-assistance was used [42]. Yu et al. had similar findings when looking at lumbar pedicle screw placement, with 4.6% of ARSN placed screws having malposition versus a 16% malposition rate for freehand placement [43].
Advantages to conventional freehand techniques over robotic guidance and ARSN include the robustness and commonly practiced nature of the technique, the ability to experience tactile feedback when placing screws which can influence real-time decision making, reduced costs and the ability to expediently adjust screw placement if required [41]. Furthermore, to our knowledge, there are currently no prospective studies available which show a significant difference between ARSN and robotic-guided procedures in terms of efficacy and mortality. Advantages of the robot guidance are the reduced ergonomic strain of screw placement, mechanical precision, the use of minimally invasive access, execution of preprogrammed screw trajectories and the use of mechanical components to improve surgical dexterity, such as tremor filters, motion scaling and directional locks to ensure accurate pedicle screw trajectories. Robot-assisted surgery is also known to have a lower operating time and radiation exposure compared with freehand placement [44, 45]. These factors may reduce fatigue or user-dependent errors. Drawbacks of robotic systems include the possibility of mechanical failure, the additional space requirement for robotic units and imperfect sensory feedback to the user console [44]. Advantages of augmented reality platforms include the use of three-dimensional imaging for visualisation of spatial parameters to improve accuracy [44]. Furthermore, the ability to reduce the surgeons ‘extrinsic’ cognitive load, by providing the navigational information within their view of the surgical site, eliminating the need to switch attention from a screen display to the surgical site [45]. Allowing integration of preoperative imaging and the intraoperative surface may also reduce the requirement for intraoperative imaging and thereby improve operational workflow while also reducing radiation exposure [45]. Additionally, ARSN systems have enhanced portability and reduced costs when compared with bulky and expensive surgical robotic systems [45]. Combining ARSN with robotic guidance, however, has obvious benefits in terms of safer, more accurate procedures with improved outcomes, lower complication rates, reduced levels of radiation exposure and shorter operating time, particularly in the setting of minimally invasive surgery. Combining these methods has the capacity to build on the benefits of each system, while eliminating many of the possible drawbacks to each method.
Thus, it is envisioned that further clinical application of AR-assisted spine surgery may be inevitable. However, there is a need for more robust prospective clinical studies for pedicle screw placement, in addition to pre-clinical versatility and proof-of-concept studies such as cervical pedicle screw placement, tumour resection, comparison to robotic navigated systems and efficacy for percutaneous non-surgical procedures such as lumbar facet joint injections. Patient cohorts should be matched accordingly for comparative studies. Only two out of five overall clinical studies which exist in the literature compare ARSN to conventional methods [14, 26]. However, different patient populations does not allow for accurate comparison between studies. Furthermore, AR could be employed to improve the consent process by demonstrating individualised surgical approaches in a manner retainable for surgical patients, improving several aspects of spine surgery both for the patient and surgeon. Nevertheless, from our study in particular, ARSN appears a safe, accurate and efficacious method of assisting in thoracolumbar pedicle screw placement.
Conclusion
ARSN pedicle screw placement had shown its consistent accuracy across a variety of models. However, its adoption into clinical spine surgery remains in its infancy, with little known regarding the learning curve. Further robust prospective studies are needed to accurately compare to conventional methods of placement.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Vávra P, Roman J, Zonča P, Ihnát P, Němec M, Kumar J, Habib N, El-Gendi A (2017) Recent development of augmented reality in surgery: a review. J Healthc Eng. https://doi.org/10.1155/2017/4574172
Burström G, Balicki M, Patriciu A, Kyne S, Popovic A, Holthuizen R, Homan R, Skulason H, Persson O, Edström E, Terander A (2020) Feasibility and accuracy of a robotic guidance system for navigated spine surgery in a hybrid operating room: a cadaver study. Sci Rep 10(1):7522
Charles YP, Cazzato RL, Nachabe R, Chatterjea A, Steib JP, Gangi A (2021) Minimally invasive transforaminal lumbar interbody fusion using augmented reality surgical navigation for percutaneous pedicle screw placement. Clin Spine Surg 34(7):E415–E424
Elmi-Terander A, Nachabe R, Skulason H, Pedersen K, Söderman M, Racadio J, Babic D, Gerdhem P, Edström E (2018) Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine (Phila Pa 1976) 43(14):1018–1023
Elmi-Terander A, Skulason H, Söderman M, Racadio J, Homan R, Babic D, van der Vaart N, Nachabe R (2016) Surgical navigation technology based on augmented reality and integrated 3D intraoperative imaging: a spine cadaveric feasibility and accuracy study. Spine (Phila Pa 1976) 41(21):E1303–E1311
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Gertzbein SD, Robbins SE (1990) Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 15(1):11–14
Spirig JM, Roner S, Liebmann F, Fürnstahl P, Farshad M (2021) Augmented reality-navigated pedicle screw placement: a cadaveric pilot study. Eur Spine J 30(12):3731–3737
Yahanda AT, Moore E, Ray WZ, Pennicooke B, Jennings JW, Molina CA (2021) First in-human report of the clinical accuracy of thoracolumbar percutaneous pedicle screw placement using augmented reality guidance. Neurosurg Focus 51(2):1–8
Liu A, Jin Y, Cottrill E, Khan M, Westbroek E, Ehresman J, Pennington Z, Lo SL, Sciubba DM, Molina CA, Witham TF (2021) Clinical accuracy and initial experience with augmented reality-assisted pedicle screw placement: the first 205 screws. J Neurosurg Spine 8:1–7
Farshad M, Spirig JM, Suter D, Hoch A, Burkhard MD, Liebmann F, Farshad-Amacker NA, Fürnstahl P (2021) Operator independent reliability of direct augmented reality navigated pedicle screw placement and rod bending. N Am Spine Soc J 8:100084
Frisk H, Lindqvist E, Persson O, Weinzierl J, Bruetzel LK, Cewe P, Burström G, Edström E, Elmi-Terander A (2022) Feasibility and accuracy of thoracolumbar pedicle screw placement using an augmented reality head mounted device. Sensors (Basel) 22(2):522
Felix B, Kalatar SB, Moatz B, Hofstetter C, Karsy M, Parr R, Gibby W (2022) Augmented reality spine surgery navigation: increasing pedicle screw insertion accuracy for both open and minimally invasive spine surgeries. Spine (Phila Pa 1976) 47:865–872
Bhatt FR, Orosz LD, Tewari A, Boyd D, Roy R, Good CR, Schuler TC, Haines CM, Jazini E (2022) Augmented reality-assisted spine surgery: an early experience demonstrating safety and accuracy with 218 screws. Global Spine J 10:21925682211069320
Chang CC, Kuo CH, Chang HK, Tu TH, Fay L, Wu JC et al (2022) Augmented reality-assisted percutaneous pedicle screw instrumentation: a cadaveric feasibility and accuracy study. Appl Sci 12:5261
Dennler C, Jaberg L, Spirig J, Agten C, Götschi T, Fürnstahl P, Farshad M (2020) Augmented reality-based navigation increases precision of pedicle screw insertion. J Orthop Surg Res 15(1):174
Gibby JT, Swenson SA, Cvetko S, Rao R, Javan R (2019) Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int J Comput Assist Radiol Surg 14(3):525–535
Elmi-Terander A, Burström G, Nachabe R, Skulason H, Pedersen K, Fagerlund M, Ståhl F, Charalampidis A, Söderman M, Holmin S, Babic D, Jenniskens I, Edström E, Gerdhem P (2019) Pedicle screw placement using augmented reality surgical navigation with intraoperative 3D imaging: a first in-human prospective cohort study. Spine 44(7):517–525
Molina CA, Theodore N, Ahmed AK, Westbroek EM, Mirovsky Y, Harel R, Orru’ E, Khan M, Witham T, Sciubba DM (2019) Augmented reality-assisted pedicle screw insertion: a cadaveric proof-of-concept study. J Neurosurg Spine 31(1):139–146
Liu H, Wu J, Tang Y, Li H, Wang W, Li C, Zhou Y (2019) Percutaneous placement of lumbar pedicle screws via intraoperative CT image-based augmented reality-guided technology. J Neurosurg Spine 32:542–547
Peh S, Chatterjea A, Pfarr J, Schäfer JP, Weuster M, Klüter T, Seekamp A, Lippross S (2020) Accuracy of augmented reality surgical navigation for minimally invasive pedicle screw insertion in the thoracic and lumbar spine with a new tracking device. Spine J 20(4):629–637
Siemionow KB, Katchko KM, Lewicki P, Luciano CJ (2020) Augmented reality and artificial intelligence-assisted surgical navigation: technique and cadaveric feasibility study. J Craniovertebr Junction Spine 11(2):81–85
Gu Y, Yao Q, Xu Y, Zhang H, Wei P, Wang L (2020) A clinical application study of mixed reality technology assisted lumbar pedicle screws implantation. Med Sci Monit 10(26):e924982
Burström G, Nachabe R, Persson O, Edström E, Elmi Terander A (2019) Augmented and virtual reality instrument tracking for minimally invasive spine surgery: a feasibility and accuracy study. Spine (Phila Pa 1976) 44(15):1097–1104
Urakov TM, Wang MY, Levi AD (2019) Workflow caveats in augmented reality-assisted pedicle instrumentation: cadaver lab. World Neurosurg 126:e1449–e1455
Elmi-Terander A, Burström G, Nachabé R, Fagerlund M, Ståhl F, Charalampidis A, Edström E, Gerdhem P (2020) Augmented reality navigation with intraoperative 3D imaging vs fluoroscopy-assisted free-hand surgery for spine fixation surgery: a matched-control study comparing accuracy. Sci Rep 10(1):707
Lai M, Skyrman S, Shan C, Babic D, Homan R, Edström E, Persson O, Burström G, Elmi-Terander A, Hendriks BHW, de With PHN (2020) Fusion of augmented reality imaging with the endoscopic view for endonasal skull base surgery; a novel application for surgical navigation based on intraoperative cone beam computed tomography and optical tracking. PLoS ONE 15(1):e0227312
Skyrman S, Lai M, Edström E, Burström G, Förander P, Homan R, Kor F, Holthuizen R, Hendriks BHW, Persson O, Elmi-Terander A (2021) Augmented reality navigation for cranial biopsy and external ventricular drain insertion. Neurosurg Focus 51(2):E7
Cercenelli L, Babini F, Badiali G, Battaglia S, Tarsitano A, Marchetti C, Marcelli E (2022) Augmented reality to assist skin paddle harvesting in osteomyocutaneous fibular flap reconstructive surgery: a pilot evaluation on a 3D-printed leg phantom. Front Oncol 11:804748
McKnight R, Pean C, Buck J, Hwang J, Hsu J, Pierrie S (2020) Virtual reality and augmented reality-translating surgical training into surgical technique. Curr Rev Musculoskelet Med 13:663–674
Ponce BA, Jennings JK, Clay TB, May MB, Huisingh C, Sheppard ED (2014) Telementoring: use of augmented reality in orthopaedic education: AAOS exhibit selection. J Bone Jt Surg Am 96(10):e84
Chytas D, Malahias M, Nikolaou V (2019) Augmented reality in orthopedics: current state and future directions. Front Surg 6:38
Tsukada S, Ogawa H, Nishino M, Kurosaka K, Hirasawa N (2019) Augmented reality-based navigation system applied to tibial bone resection in total knee arthroplasty. J Exp Orthop 6:44
Cho HS, Park YK, Gupta S, Yoon C, Han I, Kim HS, Choi H, Hong J (2017) Augmented reality in bone tumour resection: an experimental study. Bone Jt Res 6(3):137–143
Chan A, Parent E, Narvacan K, San C, Lou E (2017) Intraoperative image guidance compared with free-hand methods in adolescent idiopathic scoliosis posterior spinal surgery: a systematic review on screw-related complications and breach rates. Spine J 17(9):1215–1229
Tian NF, Huang QS, Zhou P, Zhou K, Wu RK, Lou Y et al (2011) Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J 20(6):846–859
Martin JA, Regehr G, Reznick R, MacRae H, Murnaghan J, Hutchison C, Brown M (1997) Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg 84(2):273–278
Moorthy K, Munz Y, Sarker SK, Darzi A (2003) Objective assessment of technical skills in surgery. BMJ 327(7422):1032–1037
Carl B, Bopp M, Sab B, Pojskic M, Voellger B, Nimsky C (2020) Spine surgery supported by augmented reality. Spine 10(2S):41S-55S
Yoon JW, Chen RE, Kim EJ, Akinduro OO, Kerezoudis P, Han PK, Si P, Freeman WD, Diaz RJ, Komotar RJ, Pirris SM, Brown BL, Bydon M, Wang MY, Wharen JRRE, Quinones-Hinojosa A (2018) Augmented reality for the surgeon: systematic review. Int J Med Robot Comput Assist Surg 14:e1914
Fatima N, Massaad E, Hadzipasic M, Shankar GM, Shin JH (2021) Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional free-hand technique: a systematic review and meta-analysis. Spine J 21(2):181–192
Amiot LP, Lang K, Putzier M, Zippel H, Labelle H (2000) Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 25(5):606–614
Yu X, Xu L, Bi LY (2008) Spinal navigation with intra-operative 3D-imaging modality in lumbar pedicle screw fixation. Zhonghua Yi Xue Za Zhi 88(27):1905–1908 (Chinese)
Madhavan K, Kolcun JPG, Chieng LO, Wang MY (2017) Augmented-reality integrated robotics in neurosurgery: are we there yet? Neurosurg Focus 42(5):E3
Garg B, Mehta N (2022) Great expectations with augmented reality in spine surgery: hope or hype? A commentary on the article “operator independent reliability of direct augmented reality navigated pedicle screw placement and rod bending” by Farshad et al. N Am Spine Soc J 10:100117
Funding
Open Access funding provided by the IReL Consortium. The authors declare no funding disclosures or sponsors to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Youssef, S., McDonnell, J.M., Wilson, K.V. et al. Accuracy of augmented reality-assisted pedicle screw placement: a systematic review. Eur Spine J 33, 974–984 (2024). https://doi.org/10.1007/s00586-023-08094-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-08094-5