Abstract
Purpose
Neutrophile-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are the inflammatory biomarkers of the stress response. In this study, we aimed to evaluate the effects of erector spinae plane block (ESPB) on posterior lumbar decompression and stabilization by comparing NLR, PLR, postoperative pain, opioid consumption, and functional recovery between sham block and ESPB.
Methods
This was a prospective, double-blinded, randomized controlled trial in a tertiary referral hospital. Sixty patients were randomized into two equal groups, each receiving either a sham block or ESPB. The primary outcome was the NLR and PLR 12 h and 24 h after lumbar posterior decompression and stabilization. The secondary outcomes were total opioid consumption and pain score 24 h postoperatively. Also, functional recovery determined by getting out of bed, verticalization, and walking by the balcony were reviewed as secondary outcomes.
Results
Significant differences existed between the sham block and ESPB group in NLR (29.08 ± 12.29 vs. 16.97 ± 10.38; p < 0.0001) and PLR (556.77 ± 110.32 vs. 346.43 ± 117.34; p < 0.0001) 12 h after surgery. Also, there was a significant difference in NLR (p = 0.0466) and PLR (p < 0.0001) 24 h after surgery. In addition, there was a substantial difference in pain score, total opioid consumption, and functional recovery.
Conclusion
ESPB performance during spinal surgery lowers NRL and PLR ratios 12 h and 24 h after surgery. In addition, ESPB provides better analgesia and improves functional recovery compared to sham block following posterior lumbar decompression and stabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior lumbar decompression and stabilization is a surgical procedure to correct spinal instability and deformity. Spinal cord surgery is a procedure that causes severe pain in the first days after surgery. Inadequate pain management can hinder patient recovery, mobility, and recovery and increase the risk of chronic pain. In addition, insufficient control of postoperative pain could delay the early mobilization and rehabilitation, prolong the hospital stay, worsen patient satisfaction, and promote the development of persistent postoperative pain.
The conventional analgesia model based on opioids often leads to opioid-related side effects, including nausea, vomiting, itchy skin, and dizziness, which many patients cannot tolerate. By contrast, using various drugs and technologies, a multimodal analgesic (MMA) regimen better controls postoperative pain and reduces opioid-related side effects [1]. As a vital element of MMA, regional anesthesia is essential in reducing postoperative pain. In recent years, erector spinae plane block (ESPB) has received more and more attention in spine surgery [2]. ESPB inhibits dorsal pain transmission to the thoracoabdominal spinal cord. As a part of a multidisciplinary approach, ESPB has been reported to reduce opioid use and postoperative pain during spine surgery.
The neuroendocrine system is activated during surgery and anesthesia, which results in the release of neuroendocrine hormones and cytokines. Also, postoperative pain during lumbar decompression and posterior stabilization surgery describes the control of nociceptive, neuropathic, and inflammatory pain and is associated with the surgical stress response. In addition, systemic leukocytic alterations, including leukocytosis, lymphopenia, and neutrophilia, occur in response to surgery [3]. Nowadays, the neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte (PLR) ratios are widely used across many medical disciplines as readily available and reliable markers of immune response to various non-infectious stimuli [4]. NLR predicts outcomes in patients with coronary artery disease and cancer. NLR is not only affected by surgical trauma but also by the anesthetic method [5]. However, very few studies have been conducted to evaluate the effects of different methods of anesthesia on NLR [3, 6,7,8].
This study aimed to evaluate the effects of ESPB on posterior lumbar decompression and stabilization by comparing NLR, PLR, postoperative pain, opioid consumption, and functional recovery between sham block and ESPB. In addition, this is the first trial that studies the impact of ESPB on NLR and PLR in patients undergoing posterior spinal decompression and stabilization.
Methods and patients
This was a single-centered, prospective, double-blinded, randomized controlled trial in two parallel groups. The Poznan University of Medical Sciences Bioethics Committee approved the study on the 17th of June 2020, protocol number 494/20. Enrolment occurred from the 1st of July 2020 to the 31st of January 2022. The study was performed at the Department of Anesthesiology and Intensive Care in an Independent Public Health Care Institution of the Ministry of the Interior and Administration in Poznań. The study was conducted following the principles outlined in the Helsinki Declaration. Enrolment was offered preoperatively to adults undergoing primary posterior lumbar decompression and stabilization with instrumentation involving multi-levels in the lumbar region, aged > 18 years, and ASA physical status 1 or 2.
Patients were not included in this study: if they refused to participate, had a history of opioid abuse, had an infection of the puncture site, were aged < 18 years, or were assessed as having ASA > 2. Also, in cases where no instrumentation was used, patients with a surgical intervention time of less than 60 min or longer than 300 min and patients admitted to the intensive care unit during the postoperative period were excluded.
Randomization
Patients were randomly allocated to receive ultrasound-guided ESPB block, or sham block, by computer software 1:1. A researcher who was not involved in the study prepared the randomization list and concealed group assignments consecutively numbered, sealed, opaque envelopes. A consultant anesthesiologist followed management to open the envelopes shortly before the nerve block performance to reveal the group allocation and perform the procedure according to the assignment. The patients, surgeons, operating room staff, and anesthesia team were masked to the study group allocation. Group blinding unmasking occurred once the statistical analysis was complete.
All patients underwent multilevel posterior spinal decompression and stabilization under general analgesia performed by one surgical team.
Procedures
In both groups, the patients received 7,5 mg of midazolam p.o. and 8 mg of dexamethasone i.v. half an hour before the procedure, as a part of multimodal preemptive analgesia. General anesthesia with endotracheal intubation and volume-controlled ventilation (O2/Air 40:60) was induced and maintained using IV infusions of propofol 4–12 mg/kg/h, remifentanil 0,5–0,1 mcg/kg/min, titrated to achieve hemodynamic stability monitored through radial artery line, and adequate anesthetic depth (BIS, GE Healthcare, Helsinki, Finland) values between 45 and 65. In addition, the combination of intraoperative 1.0 g acetaminophen, 1.0 g metamizole, and 400 mg ibuprofen was applied as a multimodal analgesia protocol in the opioid-sparing anesthetic regimen.
After the induction of general anesthesia, bilateral, single-injection ESPBs were performed at L4 vertebral level by an experienced regional anesthesiologist (Fig. 1). In each ESPB, a 22-gauge needle (Stimuplex Ultra 360, 80 mm) was inserted into one plane of convex array ultrasound transducer longitudinally positioned across the apex of the transverse process. The hand was directed caudally at a higher level and craniocaudally at a lower level. Penetration of the fascial plane between the transverse process and the erector spinae was confirmed by hydro-location with 1–2 mL of 0.9% isotonic saline, followed by 20 ml of injections of 0.2% ropivacaine (ESPB group) or 20 ml of 0.9% isotonic saline (sham group) by using an in-plane technique (totally 40 ml).
During the procedure: The basic hemodynamic parameters, opioid/propofol consumption, after the ESP block, the somatosensory evoked potentials (SSEP), and the duration time of the surgery were monitored.
Postoperative analgesia protocol
PCA with morphine was started to maintain postoperative analgesia in both groups before the patients were taken to the recovery room at the end of surgery. The bolus dose of morphine was prepared as 0.05 mg/kg, and bolus dose drug administration was provided in each press with a lock-in time of 30 min without basal infusion. In addition, in-service follow-up intravenous acetaminophen 1.0 g 6 hourly, 1.0 g metamizole 6 hourly, and 400 mg ibuprofen 6 hourly were administered simultaneously to prevent rebound pain for both groups.
At all postoperative time points (24, 48, 72, > 72 h), patients were asked to rate perceived pain using an 11-point numerical rating scale (NRS) rating from 0 to 10 (0 indicating no pain and 10 indicating the worst pain imaginable) experienced during motion. Total opioid consumption and time to first opioid use were measured using the PCA's electronic memory. Blood samples for NLR and PLR were collected 12 h and 12 h postoperatively.
The functional recovery was assessed at 24 h using yes or no ratings. Following abilities were rated: no problems in getting out of bed, verticalization with a balcony, and unlimited walking distance with a balcony. The rehabilitation team performed the functional recovery outcome assessment, who were blinded to the group allocation.
The outcome assessment was performed by a group of two clinicians (TR, KWT) who were blinded to the group allocation.
Sample size and statistical analysis
We considered our primary hypothesis that the ESPB improves stress response to calculate the sample size. Therefore, the sample size was calculated for the paired t-test. Depending on the previous trial [15], with a two tails type I error of 0.05, power of 80%, and an effect size factor of 0.5, it should involve 58 or more subjects.
Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA).
The parametric distribution of numerical variables was evaluated using the Kołomogorov–Simirnov normality test. The t-student or Mann–Whitney U test assessed differences between groups. Categorical variables were compared with the Mann–Whitney U test, and an analysis of contingency was compared with Fisher’s exact test. A p-value < 0,05 was considered statistically significant.
Results
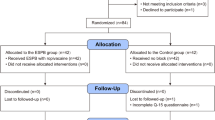
The study was conducted according to CONSORT criteria, as seen in Fig. 2. Of eighty-four patients assessed for eligibility, six did not meet the inclusion criteria, four declined to participate, and four were excluded due to a history of opioid abuse. The remaining 70 were randomly allocated between groups. Five patients did not receive the intervention due to the change of the surgical procedure. Another 5 patients were lost for follow-up, four declined to participate in the study, and one was administered to ICU, as shown in Fig. 2. The remaining sixty were analyzed. No clinically relevant differences were apparent from group characteristics, as shown in Table 1.
The results are presented in several tables to facilitate the analysis of the collected material.
Patients who underwent the ESPB had lower NLR 12 h (16.97 ± 10.38 vs. 29.08 ± 12.29; p < 0.0001) and 24 h (4.43 ± 3.22 vs. 4.43 ± 3.22; p = 0.0466) after surgery. Also, PLR was lower in the ESPB group 12 h (346.43 ± 117.34 vs. 556.77 ± 110.32; p < 0.0001) and 24 h (132.08 ± 59.61 vs. 212.53 ± 61.84; p < 0.0001), as shown in Table 2.
Patients who underwent the ESPB had lower NRS pain scores at all time points than patients in the sham block group, with a median of 3.10 (± 0.94) versus 5.34 (± 1.18) at 24 h, 5.84 (± 1.00) versus 7.52 (± 1.18) at 48 h, 5.29 (± 0.46) versus 6.52 (± 1.06) at 72 h, and 4.23 (± 0.72) versus 5.59 (± 0.95) over 72 h, all p < 0.0001 (Table 2). Every patient in the sham group received morphine intravenously due to pain treatment. In contrast, 6 (20%) in the ESPB group received none, p = 0.0237. As a result, the total opioid consumption (intravenous morphine equivalents; mg) was lower in the ESPB group at all time points 2.06(± 1.59) versus 4.45 (± 2.86), p < 0.0001 at 24 h, 0.52 (± 1.03) versus 2.55 (± 1.99), p < 0.0001 at 48 h, and 0.00 (± 0.00) versus 0.38 (± 0.78), p = 0.0326 at 72 h. In addition, the mean time to the first opioid was 8 h shorter in the sham group (p < 0,0001). The results are shown in Table 2.
There was a significant difference in functional recovery between the two groups. Therefore, on the first postoperative day, 19 (63%) patients in the ESPB group could get out of bed with no problems versus 8 (27%) patients in the sham group, p = 0.0089. Also, 14 (47%) were able to independently verticalize by the balcony versus 4 (13%), p = 0.0101, and 20 (67%) were able to walk by the balcony vs. 10 (33%), p = 0.0194. The results are shown in Table 3.
No side effects from ESPB were observed.
Discussion
In this double-blinded, randomized controlled trial (RCT) compared with sham block in the lumbar decompression and stabilization surgery, the ESPB provided less stress response expressed by the NLR and PLR, reduced pain ratings and total opioid consumption, and improved functional recovery.
ESPB suppresses the formation of proinflammatory cytokines related to the stress response [9]. Also, ESPB provides a sympathetic and inflammatory response that occurs perioperatively due to increased blood flow, vascular permeability, and leukocyte accumulation. NLR and PLR are calculated from neutrophil, platelet, and lymphocyte values from complete blood count, one of the most frequently ordered tests from clinical laboratories. They are the inflammatory indicators to predict mortality, morbidity, and subclinical inflammation [10]. NLR and PLR reflect a dynamic online relationship between adaptive (lymphocytes) and innate (neutrophils) cellular responses during different pathological states. NLR above 3.0 suggests pathological conditions like infection, inflammation, stress, and cancer [10, 11]. The severity of critical illness and stress levels are expressed by increasing NLR values [11]. Increased NLR is associated with an increased risk of mortality [12].
NLR independently correlates with surgical site infection (SSI) [13]. SSI is the third most common complication after spinal surgery. The incidence of wound infection in spinal surgery reaches up to 16% [14] and still causes significant morbidity and mortality after spine surgery [15]. Many studies showed that SSI following posterior lumbar surgery was associated with higher NLR values [16]. In our research, ESPB was associated with lower NLR values at 12 and 24 h after surgery. In addition, what is more important, after 24 h, NLR was < 3.0 in the ESPB group, in contrast to the sham group, where, after 24 h, the NLR was > 4.4. This study suggests ESPB is associated with less stress response and might be associated with a lower risk of surgical site infection, which is essential in spine surgery. Until today, no studies describe the influence of ESPB on NLR and PLR. However, Tantri et al. [9] showed that ESPB in posterior lumbar decompression and stabilization procedure was associated with low levels of interleukin-6 (Il-6) and interleukin (Il-10), two proinflammatory cytokines. Also, Liu et al. [17] showed that Il-6, Il-10, and tumor necrosis factor-alpha (TNF-α) were significantly lower in the ESB group in patients after video-assisted thoracoscopic surgery. ESPB blocks the afferent nociceptive stimulation of the injury site and enhances the effect of intravenous analgesia. This could be why the ESPB lowers stress response expressed through lower NLR, PLR, and cytocines.
ESPB provides adequate pain management with fewer side effects by blocking the dorsal and central branches of the spinal nerves. The ESPB also has a wide range of cranial and caudal spread through the paraspinal muscles via a single injection, facilitating ESPB coverage at multiple vertebral levels [18]. Therefore, based on the previous study [18], we chose the L4 vertebral level as the local anesthetic injection site [2]. Also, based on the cadaveric studies [19], the local anesthetic was injected between the deep fascia of the ESP muscle and the transverse process to cover both ventral and dorsal rami of multiple spinal nerves above and below the injection site [20].
We decided to do ESPB before surgery based on previous studies [21, 22]. The reason for using this technique is that the preoperative ultrasound anatomy is not affected, and clear images can be obtained. Also, the distribution of local anesthetic to the target area may be affected because of postoperative impaired tissue integrity [23]. Similar to other studies [24], it was observed that satisfactory analgesia was obtained after the surgery. Thus, the surgery did not affect the ropivacaine, and the bleeding during surgery or rinsing at the end of the surgery did not dilute the ropivacaine from the paravertebral space. This confirmed that the ESPB performed before the surgery may be suitable for multilevel spinal surgery.
Our study showed that ESPB reduced postoperative pain, improved NRS scores, and provided a longer duration of the first opioid administration in patients undergoing spine surgery. Also, opioid consumption was much lower in the ESPB group, essential for reducing cardio-cerebrovascular complications, particularly for patients with severe preoperative comorbidities.
Similar to our trial, Oh et al. [24], in his systemic review and meta-analysis, showed that ESBP provided adequate postoperative analgesia resulting in better satisfaction and recovery in patients undergoing lumbar surgery compared to the control group. Likewise, Rizkalla et al. [25], in the systematic review, proved that ESPB is an effective method to relieve pain after lumbar surgery.
Posterior lumbar decompression and stabilization surgery is one of the most painful surgical procedures. In addition, the pain delays early postoperative mobilization and increases the complications of infection and thrombosis. Nevertheless, it is essential because the incidence of venothromboembolic complications after spine surgery is up to 31% [26].
Also, in our study, ESPB enhanced functional recovery. For example, more than half of the patients could independently leave their beds and walk by the balcony after spinal surgery compared to less than 30% in the sham group. Similar to our study, Yao et al. [27] showed that preoperative single-injection thoracic ESPB with ropivacaine improves functional recovery in patients after video-assisted thoracic surgery.
We did not observe the side effects following ESPB, similar to other studies [28]. However, in opposition to some case reports, Elkoundi et al. [29] reported priapism developed after ESPB in treating complex regional pain syndrome (CRPS). Also, Selvi et al. [30] reported unexpected motor weakness as a side effect of the ESPB in a 29-year-old patient after a cesarean delivery operation.
The main limitation of this study was the small sample size and the volume of local anesthetic. Also, the dermatome levels were not evaluated. Another limitation was that we did not obtain NLR and PLR over 24 h. Also, the sensory block was not assessed, and the duration of the block was not reviewed. Finally, we did not monitor the hospital discharge times.
Conclusion
Based on our findings, ultrasound-guided ESPB at the low lumbar level lowers NLR and PLR, thus reducing the stress response. Also, ESPB is effective for postoperative analgesia, can reduce opioid consumption, and enhances functional recovery in patients undergoing lumbar spine surgery. Therefore, we strongly recommend this technique as a part of multimodal analgesia protocols in spine surgery.
Availability of data and material
The study datasets are available from the corresponding author at reasonable request.
References
Daoust R, Paquet J, Cournoyer A, Piette É, Morris J, Lessard J et al (2020) Side effects from opioids used for acute pain after emergency department discharge. Am J Emerg Med 38(4):695–701
Tulgar S, Aydin ME, Ahiskalioglu A, De Cassai A, Gurkan Y (2020) Anesthetic techniques: focus on lumbar erector spinae plane block. Local Reg Anesth. 13:121–133
Surhonne N, Hebri C, Kannan S, Duggappa DR, Rs RR, Mapari CG (2019) The effect of anesthetic techniques on neutrophil to lymphocyte ratio in patients undergoing infraumbilical surgeries. Korean J Anesthesiol 72(5):458–465
Kriplani A, Pandit S, Chawla A, de la Rosette JJ, Laguna P, Jayadeva Reddy S et al (2022) Neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and lymphocyte–monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis 50(3):341–348
Li Y, Li J, Cui X, Chen T, Jiang X (2023) Pre-and post-operative variation of neutrophil-to-lymphocyte ratio (NLR) predict prognosis factor in rectal cancer patients with radical primary tumor resection: a monoinstitutional retrospective study
Dağlıš SS, Dağlı R. The effect of anesthesia method on the distribution of leukocyte elements and neutrophil/lymphocyte ratio in elective cesareane sections.
Argun G, Hizli F, Duvarci M, Karaismailoglu E, Basar H, Unver S (2021) The effect of general and regional anesthesia on the neutrophil/lymphocyte and platelet/lymphocyte ratios during bladder tumor surgery. Retrosp Study
Erbaş M, Toman H, Gencer M, Şahin H, Kiraz HA, Şimşek T, et al (2019) The effect of general and spinal anesthesia on neutrophil to lymphocyte ratio in patients undergoing cesarian section. Anaesth Pain Intensive Care:485–8
Tantri AR, Rahmi R, Marsaban AHM, Satoto D, Rahyussalim AJ, Sukmono RB (2023) Comparison of postoperative IL-6 and IL-10 levels following erector spinae plane block (ESPB) and classical thoracolumbar interfascial plane (TLIP) block in a posterior lumbar decompression and stabilization procedure: a randomized controlled trial. BMC Anesthesiol 23(1):1–8
Duran H, Alpdemir M, Çeken N, Alpdemir MF, Kula AT (2022) Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections. Turk J Biochem 47(6):756–762
Zahorec R (2021) Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 122(7):474–488
Regolo M, Vaccaro M, Sorce A, Stancanelli B, Colaci M, Natoli G et al (2022) Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. J Clin Med 11(8):2235
Liu Y, Li X, Du X, Li S, Hu S, Yu Y et al (2019) Predictive value of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in surgical site infection following radical resection for rectal cancer. Med J Chin Peoples Lib Army. 44(3):243–247
Zhou J, Wang R, Huo X, Xiong W, Kang L, Xue Y (2020) Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine 45(3):208–216
Aleem IS, Tan LA, Nassr A, Riew KD (2020) Surgical site infection prevention following spine surgery. Glob Spine J. 10(1_suppl):92S-98S
Inose H, Kobayashi Y, Yuasa M, Hirai T, Yoshii T, Okawa A (2020) Postoperative lymphocyte percentage and neutrophil–lymphocyte ratio are useful markers for the early prediction of surgical site infection in spinal decompression surgery. J Orthop Surg 28(2):2309499020918402
Liu L, Ni X, Zhang L, Zhao K, Xie H, Zhu J (2021) Effects of ultrasound-guided erector spinae plane block on postoperative analgesia and plasma cytokine levels after uniportal VATS: a prospective randomized controlled trial. J Anesth. 35:3–9
Ciftci B, Ekinci M, Celik EC, Yayik AM, Aydin ME, Ahiskalioglu A (2020) Ultrasound-guided erector spinae plane block versus modified-thoracolumbar interfascial plane block for lumbar discectomy surgery: a randomized, controlled study. World Neurosurg 144:e849–e855
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ (2016) The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 41(5):621–627
Wang L, Wu Y, Dou L, Chen K, Liu Y, Li Y (2021) Comparison of two ultrasound-guided plane blocks for pain and postoperative opioid requirement in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. Pain Ther 10:1331–1341
Chin KJ, Lewis S (2019) Opioid-free analgesia for posterior spinal fusion surgery using erector spinae plane (ESP) blocks in a multimodal anesthetic regimen. Spine 44(6):E379–E383
Zhang Q, Wu Y, Ren F, Zhang X, Feng Y (2021) Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth 68:110090
Yayik AM, Cesur S, Ozturk F, Ahiskalioglu A, Ay AN, Celik EC et al (2019) Postoperative analgesic efficacy of the ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal decompression surgery: a randomized controlled study. World Neurosurg 126:e779–e785
Oh SK, Lim BG, Won YJ, Lee DK, Kim SS (2022) Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth 78:110647
Rizkalla JM, Holderread B, Awad M, Botros A, Syed IY (2021) The erector spinae plane block for analgesia after lumbar spine surgery: a systematic review. J Orthop 24:145–150
Solaru S, Alluri RK, Wang JC, Hah RJ (2021) Venous thromboembolism prophylaxis in elective spine surgery. Glob Spine J 11(7):1148–1155
Yao Y, Fu S, Dai S, Yun J, Zeng M, Li H et al (2020) Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth 63:109783
Guna Pratheep K, Sonawane K, Rajasekaran S, Shetty AP, Subramanian BJ, Kanna RM (2021) Transient paraplegia in lumbar spine surgery—a potential complication following erector spinae plane block. Eur Spine J, pp 1–5
Elkoundi A, Bentalha A, El Kettani SEC, Mosadik A, El Koraichi A (2019) Erector spinae plane block for pediatric hip surgery-a case report. Korean J Anesthesiol 72(1):68–71
Selvi O, Tulgar S (2018) Bloqueo en el plano del erector de la columna ecoguiado como causa de bloqueo motor imprevisto. Rev Esp Anestesiol Reanim 65(10):589–592
Acknowledgements
Not applicable.
Funding
Funding was provided by Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
Author information
Authors and Affiliations
Contributions
All of the authors (MD, BC, TR, JR, KWT, GK) made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; they have been involved in drafting the manuscript or revising it critically for important intellectual content; have given final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
The Poznan University of Medical Sciences Bioethics Committee approved the study on the 17th of June 2020, protocol number 494/20.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Domagalska, M., Ciftsi, B., Janusz, P. et al. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) levels following erector spinae plane block (ESPB) in posterior lumbar decompression: a randomized, controlled trial. Eur Spine J 32, 4192–4199 (2023). https://doi.org/10.1007/s00586-023-07913-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07913-z