Abstract
Purpose
Spinal cavernous malformations (SCM) present a risk for intramedullary hemorrhage (IMH), which can cause severe neurologic deficits. Patient selection and time of surgery have not been clearly defined.
Methods
This observational study included SCM patients who underwent surgery in our department between 2003 and 2021. Inclusion required baseline clinical factors, magnetic resonance imaging studies, and follow-up examination. Functional outcome was assessed using the Modified McCormick scale score.
Results
Thirty-five patients met the inclusion criteria. The mean age was 44.7 ± 14.5 years, and 60% of the patients were male. In univariate analysis, the unfavorable outcome was significantly associated with multiple bleeding events (p = .031), ventral location of the SCM (p = .046), and incomplete resection (p = .028). The time between IMH and surgery correlated with postoperative outcomes (p = .004), and early surgery within 3 months from IMH was associated with favorable outcomes (p = .033). This association remained significant in multivariate logistic regression analysis (p = .041).
Conclusions
Removal of symptomatic SCM should be performed within 3 months after IMH when gross total resection is feasible. Patients with ventrally located lesions might be at increased risk for postoperative deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous malformations of the spinal cord (SCM) are rare neurovascular low-flow lesions that, like cerebral cavernous malformations (CCM), can cause parenchymal hemorrhage [1, 2]. Depending on the malformation's location and the hemorrhage's size, SCM may cause severe neurologic deficits. Therefore, surgical treatment should be considered, especially in cases of good accessibility and a history of recurrent bleeding [3,4,5,6,7]. However, current precise management guidelines (surgical versus conservative treatment) are lacking because cavernous malformations of the central nervous system are rare and affect a very heterogeneous patient population. Recently published data suggested that SCM have both a more aggressive course than CCM and that each bleeding event decreases the likelihood of neurological recovery [8,9,10,11,12]. For these reasons, there is an urgent need to investigate whether the benefits of early surgical treatment may outweigh the natural course of SCM and its bleeding-associated impairment.
There is increasing evidence that surgical intervention leads to better outcomes for most patients with symptomatic intramedullary hemorrhage (IMH) [3,4,5,6,7]. Nevertheless, some disagreement remains with considerable controversial opinions, such as the perfect timing for surgical intervention [4, 13] or indications for resection of deep-seated lesions [4, 14, 15]. Due to the rare nature of SCM, there are few neurosurgical centers with enough case volume in SCM management. Therefore, it is imperative to provide collective outcome data to clarify these open questions to reach enough evidence for future treatment guidelines.
In this study, we aimed to determine both the functional postoperative outcome after SCM removal and outcome predictors.
Methods
Study design
This observational study was conducted at our tertiary university hospital, with approval from our institutional review board (14-5751-BO and 19-8662-BO), as well as with the Declaration of Helsinki principles. We conducted a patient registry database including all patients treated surgically with SCM in our department from 2003 until 2021. Inclusion regarded patients with available medical records and baseline health metrics, magnetic resonance imaging (MRI) studies, and at least one follow-up examination. The decision for surgery was made on a case-by-case basis and after a discussion at a multidisciplinary neurovascular conference.
Data collection
Baseline health metrics such as age at diagnosis, sex, and SCM location were obtained through patient chart review. Follow-up data included routine neurological examinations in a specialized outpatient clinic. Sagittal and axial plane T2-weighted images were used to determine SCM location. IMH was defined according to reporting standards as follows: acute or subacute onset of neurological symptoms that are related to the anatomical location with confirmation of acute bleeding on a recent MRI scan [16]. The final decision to assign a patient to the symptomatic hemorrhage group was ultimately decided through consensus by authors LR, YL, and PD based on clinical examination and MRI. Neurological functional status was classified using the Modified McCormick (MMcC) scale score assessed at diagnosis, at the time of IMH, at postoperative discharge, and at the last follow-up examination [17]. A more than one point increase in MMcC rating was defined as an unfavorable functional outcome. According to the MMcC classification (I–V), the functional neurological status of each patient was assessed at different time points using one of the following score categories: (I) neurologically intact with normal ambulation, may have minimal dysesthesia; (II) mild motor or sensory deficit and functional independence; (III) moderate deficit and limitation of function but independent with external aid; (IV) severe motor or sensory deficit with limited function and dependent on external aid; or (V) paraplegia or quadriplegia, even if there is flickering movement.
Statistical analysis and illustration of cases
The primary aim of this study was to analyze the functional outcome after the surgical removal of the SCM. The secondary aim was to assess predictors of worse functional outcomes. We used SPSS-27 software for all statistical analyses. Univariate analyses were performed to determine predictors of unfavorable outcomes at the last follow-up. For dichotomized variables, the Chi-square test (sample size > 5) or the Fisher exact test (sample size ≤ 5) was used. Continuous variables were tested with the student’s t test (normally distributed data) or Mann–Whitney-U test (non-normally distributed data). The degree of correlation was calculated using Spearman’s rank correlation test. Significant associations were assessed in a multivariable logistic regression analysis to identify independent predictors of unfavorable outcomes at the last follow-up. All tests were two-tailed, and p values < .05 were defined as significant. The illustrations were created using BioRender software.
Results
Patient demographics
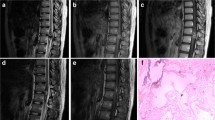
Seventy-five patients with SCM were identified out of 1492 patients with central nervous system cavernous malformation in our database. Of those, 35 underwent surgery, some after trying conservative therapy. All 35 patients fulfilled the above-mentioned inclusion criteria and were eligible for this study. In the final cohort, the mean age was 44.7 ± 14.5 years, and most patients (n = 21, 60%) were male. Most SCM were thoracic (n = 20, 57.1%). Nearly half of the lesions (n = 20, 57.1%) affected the spinal cord's ventral part, and many SCM (n = 22, 68.8%) crossed the midline. All patients in the cohort suffered from at least one symptomatic IMH and 12 patients (34.3%) experienced ≥ 2 IMH events. All patients underwent surgery, and gross total resection was achieved in 29 (82.9%) cases. Every patient with an incomplete extent of resection experienced recurrent IMH during postoperative follow-up. The mean time of follow-up was 51.4 ± 53.6 months. The average time from diagnosis to surgical treatment was 16.7 ± 34.0 since some patients underwent rehabilitation first and others received surgical removal after the second bleeding event. The average postoperative follow-up was 34.3 ± 43.4 months. Table 1 summarizes the demographic and clinical features of the study. Figure 1 illustrates exemplary cases.
Exemplary SCM cases. A Illustration of exemplary cases with different intramedullary locations. A preoperative (left) and a postoperative (right) sagittal T2-weighted MRI scan are shown in each case. B Intraoperative situs. Shown is the spinal cord after splitting the dura mater (top) and access to the bleeding cavity through a median myelotomy for a medially localized SCM (bottom)
Postoperative outcome and predictors of unfavorable postoperative outcome
Postoperative worsening was found in 14 patients (40.0%). Univariate analysis revealed that unfavorable postoperative outcomes were associated with multiple bleeding events (OR = 5.67, 95% CI = 1.24–25.88, p = .031), incomplete SCM resection (OR = 11.11, 95% CI = 1.13–109.3, p = .028), and localization of the cavernoma in the ventral part of the spinal cord (OR = 4.89, 95% CI = 1.05–22.84, p = .046). Detailed information is provided in Table 2.
We found a correlation between the timing of surgery and unfavorable outcomes (r = .497, p = .004). Also, early surgery (i.e., within 3 months after IMH) was associated with a favorable postoperative outcome (OR = 2.13, 95% CI = 1.02–4.48, p = .033). In a multivariate logistic regression analysis, this association remained significant (aOR = 5.99, 95% CI = 1.08–33.30, p = .041), while localization of the cavernoma in the ventral part of the spinal cord and incomplete SCM resection failed to be independent predictors and did not reach the necessary level of significance (p > .05). Results are illustrated in Table 3.
Discussion
SCM disease poses a significant risk for IMH and can cause severe neurological disability with an impact on physical and psychological life [9, 11]. These lesions are generally surgically accessible, and their resection has become considerably safer within the last few decades, among other things due to new insights into the spontaneous course of cavernoma disease as well as due to increased intraoperative safety by microsurgical treatment using electrophysiological monitoring [18, 19]. Although gross total resection is curative and prevents the risk of devastating deficits from recurrent bleeding events, spinal cord surgery remains risky, and the decision to operate should always be considered carefully. There is expert consensus that symptomatic patients with accessible SCM are good candidates for surgery and should undergo surgical treatment, while those who fail in any of these features may warrant conservative management [3,4,5,6,7]. Although growing evidence supports aggressive surgical treatment, specific selection criteria and operation indications are lacking in the literature.
Timing of surgery
Optimal surgical timing for SCM remains controversial. Many studies have not correlated patient outcomes with the timing of surgery, and only a few authors provide data on this relevant topic. Many authors are in favor of early resection once the decision to operate has been made [4, 6, 7, 20, 21]. On the other hand, Imagama and colleagues have published the only existing prospective study on SCM to date where they recommend waiting for neurologic rehabilitation before surgical removal [13]. This contrasts with the findings of a large meta-analysis by Badhiwala et al., who found a significant correlation between shorter duration of presurgical symptoms and favorable clinical outcomes [4]. The results of our study suggest that delaying surgical resection may be harmful.
These findings are in line with many authors and support the often-recommended practice of early SCM resection [2,3,4, 7, 19, 22,23,24]. The rationale for this recommendation in our study is the presumption that waiting longer than 3 months after IMH might increase the risk of early recurrent bleeding and irreversible myelopathy due to the IMH-related mass effect. However, extra-lesional hematoma and a gliotic plane between the cavernous malformation and spinal cord after IMH usually facilitate SCM resection, which may render surgery less traumatic to the spinal tracts [6]. Therefore, the optimal window for surgery could be between a few weeks and three months.
The extent of resection and recurrent bleeding
In almost, all studies a gross total resection was recommended, but these studies have not investigated the outcome of patients with residual cavernoma tissue. According to recently published reviews on surgical treatment of SCM, gross total resection is achievable in more than 90% of all cases [3, 5]. Our data indicate recurrent IMH in all patients that underwent incomplete resection and emphasize the urgent need for gross total resection when safe. What remains unanswered, however, is the question of how to proceed after incomplete resection. Given our data, second-look surgery may be considered to prevent recurrent hemorrhage and prevent further deterioration due to re-hemorrhage. However, given the lack of evidence, this must still be decided on a single-case basis.
Deep lesions
Since many studies do not distinguish between superficial and deep SCM, the question arises of whether both entities should be managed equally. Although surgical approaches and safe-entry zones to spinal cord lesions are well defined [4, 19], the risk of iatrogenic spinal cord injury is greater when the lesions are located in the ventral portion of the spinal cord [14]. With this in mind, some authors differentiate between superficial and deep lesions when making treatment recommendations. Notably, Gross and colleagues recommend in their study to remove exophytic lesions irrespective of the clinical presentation, to observe deep-seated asymptomatic lesions, and to remove deep-seated symptomatic lesions only in cases of severe or progressive symptoms [5]. A similar approach is recommended by Liang and colleagues, who preferer a watch-and-wait approach in small and ventrally located SCM [15]. The results of our study emphasize the need for differentiated management relative to anatomical location. Our study indicates that ventrally located SCM have a higher risk for an unfavorable postoperative outcome and that surgery of such lesions must be favored when safe.
External validity
Compared to other studies analyzing patients suffering from SCM such as Goyal and colleagues, which is the largest single-center series available, our cohort was similar in terms of baseline health features, including 45% versus 40% females, mean age of 49.6 ± 17.3 versus 44.7 ± 14.5 years, and the majority of lesions located in the thoracic spine accounting for 59% versus 57.1% of patients accordingly [9]. These similarities increase the external validity of our results.
Limitations
One major drawback of our study is the use of retrospective data, limiting the evidence level and the generalization of our results. Moreover, the data of our study are not population-based and was acquired from a tertiary referral center, which can lead to information and selection biases. Due to the rarity of SCM disease, the number of cases in this study was small. The lack of power in our sample may account for loss of statistical significance in the multivariate analysis.
Perspective
IMH and spinal cord surgery inherently bear the risk of transient and permanent injury (e.g., weakness, loss of coordination, sensory deficits, or bowel and bladder dysfunction). Gross total resection is the only certain prevention of (re)bleeding, but implies immediate (e.g., infection, deep vein thrombosis, and cerebrospinal fluid leakage) or late (e.g., kyphosis, tethered cord, stenosis, SCM rebleed, and subsequent surgery) risks [25]. For this reason, conservative and surgical approaches must be well balanced against each other, and the final decision should be made on a case-by-case basis in experienced neurovascular centers. Our study contributes novel data to this rare disease and underlines the value of early surgery while highlighting the need for personalized decision-making. There is an unmet need for further studies, especially prospective multicenter studies with a large number of patients, to validate our results and to enable the creation of guidelines.
Conclusion
The results of this work emphasize the importance of early surgical removal of hemorrhagic and symptomatic SCM. If gross total resection can be achieved with acceptable morbidity, patients should be operated on within 3 months after IMH. Patients with deep-seated lesions might be at higher risk for postoperative deficits, and the indication for surgery should be carefully considered.
Data availability
All data for this study are available from the corresponding author upon reasonable request.
References
Awad IA, Polster SP (2019) Cavernous angiomas: deconstructing a neurosurgical disease. J Neurosurg 131:1–13. https://doi.org/10.3171/2019.3.Jns181724
Otten M, McCormick P (2017) Natural history of spinal cavernous malformations. Handb Clin Neurol 143:233–239. https://doi.org/10.1016/b978-0-444-63640-9.00022-9
Asimakidou E, Meszaros LT, Anestis DM, Tsitsopoulos PP (2022) A systematic review on the outcome of intramedullary spinal cord cavernous malformations. Eur Spine J. https://doi.org/10.1007/s00586-022-07332-6
Badhiwala JH, Farrokhyar F, Alhazzani W, Yarascavitch B, Aref M, Algird A, Murty N, Kachur E, Cenic A, Reddy K, Almenawer SA (2014) Surgical outcomes and natural history of intramedullary spinal cord cavernous malformations: a single-center series and meta-analysis of individual patient data: clinic article. J Neurosurg Spine 21:662–676. https://doi.org/10.3171/2014.6.Spine13949
Gross BA, Du R, Popp AJ, Day AL (2010) Intramedullary spinal cord cavernous malformations. Neurosurg Focus 29:E14. https://doi.org/10.3171/2010.6.Focus10144
Jallo GI, Freed D, Zareck M, Epstein F, Kothbauer KF (2006) Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus 21:e10. https://doi.org/10.3171/foc.2006.21.1.11
Velz J, Bozinov O, Sarnthein J, Regli L, Bellut D (2018) The current management of spinal cord cavernoma. J Neurosurg Sci 62:383–396. https://doi.org/10.23736/s0390-5616.18.04305-9
Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP (2012) Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 11:217–224. https://doi.org/10.1016/s1474-4422(12)70004-2
Goyal A, Rinaldo L, Alkhataybeh R, Kerezoudis P, Alvi MA, Flemming KD, Williams L, Diehn F, Bydon M (2019) Clinical presentation, natural history and outcomes of intramedullary spinal cord cavernous malformations. J Neurol Neurosurg Psychiatry 90:695–703. https://doi.org/10.1136/jnnp-2018-319553
Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D, Maxwell SS, White P, Christianson TJ, Agid R, Cho WS, Oh CW, Wu Z, Zhang JT, Kim JE, Ter Brugge K, Willinsky R, Brown RD Jr, Murray GD, Al-Shahi Salman R (2016) Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol 15:166–173. https://doi.org/10.1016/s1474-4422(15)00303-8
Kharkar S, Shuck J, Conway J, Rigamonti D (2007) The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery 60:865–872. https://doi.org/10.1227/01.Neu.0000255437.36742.15
Santos AN, Rauschenbach L, Darkwah Oppong M, Gembruch O, Saban D, Chen B, Herten A, Schmidt B, Li Y, Özkan N, Jabbarli R, Wrede K, Sure U, Dammann P (2021) Natural course of untreated spinal cord cavernous malformations: a follow-up study within the initial 5 years after diagnosis. J Neurosurg Spine. https://doi.org/10.3171/2021.9.Spine211052
Imagama S, Ito Z, Ando K, Kobayashi K, Hida T, Ito K, Tsushima M, Ishikawa Y, Matsumoto A, Morozumi M, Tanaka S, Machino M, Ota K, Nakashima H, Wakao N, Sakai Y, Matsuyama Y, Ishiguro N (2017) Optimal timing of surgery for intramedullary cavernous hemangioma of the spinal cord in relation to preoperative motor paresis, disease duration, and tumor volume and location. Global Spine J 7:246–253. https://doi.org/10.1177/2192568217707938
Labauge P, Bouly S, Parker F, Gallas S, Emery E, Loiseau H, Lejeune JP, Lonjon M, Proust F, Boetto S, Coulbois S, Auque J, Boulliat J (2008) Outcome in 53 patients with spinal cord cavernomas. Surg Neurol 70:176–181. https://doi.org/10.1016/j.surneu.2007.06.039
Liang JT, Bao YH, Zhang HQ, Huo LR, Wang ZY, Ling F (2011) Management and prognosis of symptomatic patients with intramedullary spinal cord cavernoma. J Neurosurg Spine 15:447–456. https://doi.org/10.3171/2011.5.Spine10735
Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA (2008) Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Sci Advisory Board Stroke 39:3222–3230. https://doi.org/10.1161/strokeaha.108.515544
Manzano G, Green BA, Vanni S, Levi AD (2008) Contemporary management of adult intramedullary spinal tumors-pathology and neurological outcomes related to surgical resection. Spinal Cord 46:540–546. https://doi.org/10.1038/sc.2008.51
Bian LG, Bertalanffy H, Sun QF, Shen JK (2009) Intramedullary cavernous malformations: clinical features and surgical technique via hemilaminectomy. Clin Neurol Neurosurg 111:511–517. https://doi.org/10.1016/j.clineuro.2009.02.003
Mitha AP, Turner JD, Spetzler RF (2011) Surgical approaches to intramedullary cavernous malformations of the spinal cord. Neurosurgery 68:317–324. https://doi.org/10.1227/NEU.0b013e3182138d6c
Aoyama T, Hida K, Houkin K (2011) Intramedullary cavernous angiomas of the spinal cord: clinical characteristics of 13 lesions. Neurol Med Chir 51:561–566. https://doi.org/10.2176/nmc.51.561
Steiger HJ, Turowski B, Hänggi D (2010) Prognostic factors for the outcome of surgical and conservative treatment of symptomatic spinal cord cavernous malformations: a review of a series of 20 patients. Neurosurg Focus 29:E13. https://doi.org/10.3171/2010.6.Focus10123
Li J, Chen G, Gu S, Liu X, Shou J, Gu W, Gao X, Xu Q, Che X, Xie R (2018) Surgical outcomes of spinal cord intramedullary cavernous malformation: a retrospective study of 83 patients in a single center over a 12-Year period. World Neurosurg 118:e105–e114. https://doi.org/10.1016/j.wneu.2018.06.134
Lu DC, Lawton MT (2010) Clinical presentation and surgical management of intramedullary spinal cord cavernous malformations. Neurosurg Focus 29:E12. https://doi.org/10.3171/2010.6.Focus10139
Tong X, Deng X, Li H, Fu Z, Xu Y (2012) Clinical presentation and surgical outcome of intramedullary spinal cord cavernous malformations. J Neurosurg Spine 16:308–314. https://doi.org/10.3171/2011.11.Spine11536
Mitha AP, Turner JD, Abla AA, Vishteh AG, Spetzler RF (2011) Outcomes following resection of intramedullary spinal cord cavernous malformations: a 25-year experience. J Neurosurg Spine 14:605–611. https://doi.org/10.3171/2011.1.Spine10454
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study did not receive special funding.
Author information
Authors and Affiliations
Contributions
None.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rauschenbach, L., Santos, A.N., Engel, A. et al. Functional neurological outcome of spinal cavernous malformation surgery. Eur Spine J 32, 1714–1720 (2023). https://doi.org/10.1007/s00586-023-07640-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07640-5