Abstract
Purpose
To describe long-term outcomes of conservative treatment for chronic coccydynia.
Methods
We conducted a 36-month prospective observational study. Adults with chronic coccydynia (> 2 months) were included. The first-line treatment was coccygeal corticosteroid injection. The second-line treatment was either manual therapy or coccygectomy. The primary endpoint was the mean variation from baseline of coccydynia intensity at 6 and 36 months, using a numeric rating scale (0, no pain; 10, maximal pain). Evolution was considered unfavorable when coccydynia intensity was > 3 of 10 points at 36 months or coccygectomy had been performed. We carried out bivariate and multivariate analyses to identify variables associated with an unfavorable evolution.
Results
We included 115 participants. Mean (SD) age was 43.5 (12.3) years, duration of coccydynia 18.4 (21.6) months and coccydynia intensity 6.5 (2.0) of 10 points. Mean variations for coccydynia intensity were − 1.5 (3.0) at 6 months and − 2.8 (3.2) at 36 months. At 36 months, 59/115 (51%) participants had an unfavorable evolution. In bivariate analysis, posterior coccyx dislocations were numerically more frequent in participants with an unfavorable evolution compared to others (29/59 (48%) versus 17/56 (30%), p = 0.057). In multivariate analysis, longer duration of coccydynia was associated with an unfavorable evolution (OR = 1.04, 95% CI from 1.01 to 1.07, p = 0.023).
Conclusion
In adults with chronic coccydynia receiving conservative treatment, symptoms decrease overtime, but significantly persist at 36 months in more than half of them. For patients with posterior coccyx dislocation, coccygectomy may be considered rapidly.

Similar content being viewed by others
References
Maigne JY, Tamalet B (1996) Standardized radiologic protocol for the study of common coccygodynia and characteristics of the lesions observed in the sitting position. Clinical elements differentiating luxation, hypermobility, and normal mobility. Spine 21:2588–2593. https://doi.org/10.1097/00007632-199611150-00008
Doursounian L, Maigne JY, Faure F, Chatellier G (2004) Coccygectomy for instability of the coccyx. Int Orthop 28:176–179. https://doi.org/10.1007/s00264-004-0544-3
Hofstetter CP, Brecker C, Wang MY (2015) Coccygectomy: current views and controversies. Contemp Spine Surgery 16:1–5. https://doi.org/10.1097/01.css.0000462791.73066.41
Kerr EE, Benson D, Schrot RJ (2011) Coccygectomy for chronic refractory coccygodynia: clinical case series and literature review. J Neurosurg Spine 14:654–663. https://doi.org/10.3171/2010.12.spine10262
Kleimeyer JP, Wood KB, Lønne G, Herzog T, Ju K, Beyer L, Park C (2017) Surgery for refractory coccygodynia: operative versus nonoperative treatment. Spine 42:1214–1219. https://doi.org/10.1097/brs.0000000000002053
Wray CC, Easom S, Hoskinson J (1991) Coccydynia. Aetiology and treatment. J Bone Joint Surg Br 73:335–338. https://doi.org/10.1302/0301-620x.73b2.2005168
Maigne JY, Guedj S, Straus C (1994) Idiopathic coccygodynia. Lateral roentgenograms in the sitting position and coccygeal discography. Spine 19:930–934. https://doi.org/10.1097/00007632-199404150-00011
Maigne JY, Doursounian L, Chatellier G (2000) Causes and mechanisms of common coccydynia: role of body mass index and coccygeal trauma. Spine 25:3072–3079. https://doi.org/10.1097/00007632-200012010-00015
Maigne JY, Chatellier G, Faou ML, Archambeau M (2006) The treatment of chronic coccydynia with intrarectal manipulation: a randomized controlled study. Spine 31:E621-627. https://doi.org/10.1097/01.brs.0000231895.72380.64
Howard PD, Dolan AN, Falco AN, Holland BM, Wilkinson CF, Zink AM (2013) A comparison of conservative interventions and their effectiveness for coccydynia: a systematic review. J Man Manip Ther 21:213–219. https://doi.org/10.1179/2042618613y.0000000040
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. https://doi.org/10.1016/s0140-6736(07)61602-x
Koop PM, Strang V (2002) Prospective and retrospective research: considerations and questions. Can Oncol Nurs J 12:142–145
Daste C, Abdoul H, Foissac F, Lefèvre-Colau MM, Poiraudeau S, Rannou F, Nguyen C (2020) Patient acceptable symptom state for patient-reported outcomes in people with non-specific chronic low back pain. Ann Phys Rehabil Med. https://doi.org/10.1016/j.rehab.2020.10.005
Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, Felson DT, Hajjaj-Hassouni N, Hochberg M, Logeart I, Matucci-Cerinic M, van de Laar M, van der Heijde D, Dougados M (2012) Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: Results from a prospective multinational study. Arthritis Care Res (Hoboken) 64:1699–1707. https://doi.org/10.1002/acr.21747
Hodges SD, Eck JC, Humphreys SC (2004) A treatment and outcomes analysis of patients with coccydynia. Spine J 4:138–140. https://doi.org/10.1016/j.spinee.2003.07.011
Mitra R, Cheung L, Perry P (2007) Efficacy of fluoroscopically guided steroid injections in the management of coccydynia. Pain Physician 10:775–778
Funding
None.
Author information
Authors and Affiliations
Contributions
SC, JYM, CN contributed to conception and design of the study. JYM and CN contributed to drafting of the original protocol. CN contributed to design of the statistical analysis plan. JYM contributed to coordination of the study. JYM contributed to acquisition of data. SC, JYM and CN contributed to drafting of the present manuscript. EC, MMLC and FR contributed to reviewing and providing comments on the manuscript. SC, JYM, EC, MMLC, FR and CN contributed to final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethics approval (include appropriate approvals or waivers).
Our study protocol was approved by our ethics committee (CERAP-HP.5).
Consent to participate (include appropriate statements)
All participants in the study provided informed consent.
Consent for publication (include appropriate statements)
All authors listed provided final written approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of data and material (data transparency)
Academic researchers can request access to data and material by contacting Associate Professor Christelle Nguyen at christelle.nguyen2@aphp.fr.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement
Item No | Recommendation | Page No | |
|---|---|---|---|
Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
(b) Provide in the abstract an informative and balanced summary of what was done and what was found | 3 | ||
Introduction | |||
Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4 |
Objectives | 3 | State specific objectives, including any prespecified hypotheses | 4 |
Methods | |||
Study design | 4 | Present key elements of study design early in the paper | 4 |
Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 4 |
Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case–control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants | 4, 5 |
(b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case–control study—For matched studies, give matching criteria and the number of controls per case | NA | ||
Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 5, 6 |
Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 5–6 |
Bias | 9 | Describe any efforts to address potential sources of bias | NA |
Study size | 10 | Explain how the study size was arrived at | NA |
Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 6 |
Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 6 |
(b) Describe any methods used to examine subgroups and interactions | NA | ||
(c) Explain how missing data were addressed | NA | ||
(d) Cohort study—If applicable, explain how loss to follow-up was addressed Case–control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy | NA | ||
(e) Describe any sensitivity analyses | NA | ||
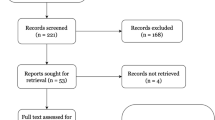
Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 6, Appendix 4 |
(b) Give reasons for non-participation at each stage | 6, Appendix 4 | ||
(c) Consider use of a flow diagram | 6, Appendix 4 | ||
Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 6, Table 1 |
(b) Indicate number of participants with missing data for each variable of interest | |||
(c) Cohort study—Summarise follow-up time (eg, average and total amount) | NA | ||
Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | Table 2 |
Case–control study—Report numbers in each exposure category, or summary measures of exposure | NA | ||
Cross-sectional study—Report numbers of outcome events or summary measures | NA | ||
Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Table 2 |
(b) Report category boundaries when continuous variables were categorized | NA | ||
(c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | NA | ||
Other analyses | 17 | Report other analyses done—e.g. analyses of subgroups and interactions, and sensitivity analyses | NA |
Key results | 18 | Summarise key results with reference to study objectives | 8 |
Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 8, 9 |
Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 8, 9, 10 |
Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 8, 9 |
Other information | |||
Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 13 |
Appendix 2 Paris self-administered questionnaire. Score ranging from 0 (no symptoms) to 10 (maximal symptoms)
Circle the number which best describes your response. To ensure that your questionnaire will count, please answer all 6 questions.

Appendix 3 Modified Dallas self-administered questionnaire. Score ranging from 0 (no symptoms) to 30 (maximal symptoms)
For each question, tick the answer that best suits you.
1) Social activity. How much does your pain interfere with your social life (dancing, games and entertainment, meals or parties with friends, going out, etc.)?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
2) Professional activities. How much does your pain interfere with your work?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
3) Self-control. How well are you in control of your emotional reactions?
-
Totally (0)
-
Often (1)
-
I'm having trouble (2)
-
I can't do it (3)
4) Anxiety/morale. How well do you feel you are dealing with what is required of you?
-
Totally (0)
-
Often (1)
-
I'm having trouble (2)
-
I can't do it (3)
5) Worry. How worried are you about your tailbone pain?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
6) Stress. How stressed do you feel you are about your tailbone pain?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
7) Depression. How depressed have you been since the pain?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
8) Relationships with others. How much do you think your pain has changed your relationship with others?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
9) Support from others. How much support do you need from others since your pain (housekeeping, meal preparation, etc.)?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
10) Adverse reactions of relatives. How do you feel that your pain causes irritation, annoyance, anger towards you in those around you?
-
Not at all (0)
-
A little (1)
-
Moderately (2)
-
A lot (3)
Appendix 4 Flow diagram

Rights and permissions
About this article
Cite this article
Charrière, S., Maigne, JY., Couzi, E. et al. Conservative treatment for chronic coccydynia: a 36-month prospective observational study of 115 patients. Eur Spine J 30, 3009–3018 (2021). https://doi.org/10.1007/s00586-021-06911-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-06911-3