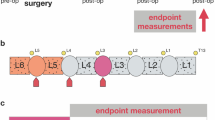
Abstract
Study design
A multi-cohort, case–control rodent study.
Purpose
Investigate the long-term behavioural, histologic and radiologic consequences on the complete lumbar spine of L4/5 intervertebral disc (IVD) injury in mice and determine if increased physical activity mitigates the observed changes.
Methods
Cohorts of 2-month-old CD1 female mice underwent a single ventral puncture of the L4/5 IVD. 0.5-, 3- or 12-months after injury, general health (body weight and locomotor capacity), behavioural signs of axial discomfort (tail suspension, grip strength and FlexMaze assays) and radiating pain (von Frey and acetone tests) were assessed. Experimental groups with free access to an activity wheel in their home cages were including in the 12-month cohort. Lumbar disc status was determined using colorimetric staining and radiologic (X-ray and T2-MRI) analysis. Innervation was measured by immunoreactivity for PGP9.5 and calcitonin gene‐related peptide.
Results
No changes in general health or persistent signs of axial discomfort were observed up to one year post-injury. In contrast, signs of radiating pain developed in injured mice at 3 months post-injury, persisted up to 12 months and were reversed by long-term physical activity. At 12-months post-injury, degeneration was observed in non-injured lumbar discs. Secondary degenerating IVDs were similar to the injured discs by X-ray (narrowing) and T2-MRI (internal disc disruption) but did not show abnormal innervation. Increased physical activity had no impact on mechanically injured IVDs, but attenuated disc narrowing at other lumbar levels.
Conclusions
Mechanical injury of L4/5-IVDs induces delayed radiating pain and degeneration of adjacent discs; increased physical activity positively mitigated both.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a leading cause of global disability [1] and current treatments either lack efficacy or are limited by undesired side-effects. Degeneration in intervertebral discs (IVDs) is one of main causes of LBP and is often diagnosed by MRI assessment. Patients often incorporate lifestyle-based interventions into their treatment such as running, yoga and aerobic fitness, all of which are shown to attenuate pain, disability or both [2]. While beneficial effects of physical activity in LBP are well documented, its impact on IVDs is not well explored.
In the present study, we used our previously reported mouse model of chronic LBP induced by a single puncture of the mouse L4/5-IVD [3, 4] to investigate the evolution of lumbar IVD degeneration over time using histological and radiologic tools and the relationship between degeneration and behavioural changes. The effect of long-term physical activity on behaviour, degeneration and innervation of injured and adjacent, non-injured IVDs was also investigated.
Materials and methods
Please see supplementary methods for additional information on animals, surgical, behavioural and radiologic procedures.
Animals
Four cohorts (i.e. 0.5 M, 3 M, 12 M, 12 M + run) of female 20 g CD1 mice were included in this study (Charles River, Canada, 3/cage), see Table 1. Behavioural and radiologic assessments and tissue harvest were performed 0.5-, 3- or 12-months following L4/5-IVD injury. 12 M + run cohort: starting 2 weeks post-injury, active mice received a home cage running wheel (dome + wheel, Bio-serve, Flemington, NJ, USA). 12 M-cohort: sedentary mice received a sham device (the dome and wheel were fixed together to prevent rotation and voluntary activity).
Mice injured at levels other than L4/5 were excluded from the study (Table 1). All experiments were approved by the Institutional Animal Care and Use Committee of McGill University and conformed to national and international ethical guidelines [5].
Surgical procedure
Injury to the L4/5-IVD was induced as described previously [3] by a single ventral mechanical puncture with a 30G needle equipped with a stopper (0.7 mm). Allocation to the sham or injured surgery was randomly assigned across cage mates using a Latin square design.
Behavioural procedures
General health
Locomotor capacity (rotarod) Latency to fall in seconds was measured using an accelerating rotarod (IITC) with the mouse adapter (rod diameter, 3.2 cm) [6].
Voluntary running Assessed using wireless Counting Wheels (ENV-047, Med associates Inc.) for 60 min.
Axial discomfort
Grip force assay Used to assess tensile strength (tolerance to axial stretching), the forearm grip strength was measured using a Grip Strength Metre (Stoelting Co, Wood Dale, IL, USA) [6].
Tail suspension assay Mice were individually suspended underneath a platform by the tail, videotaped for 180 s. Duration in immobility was quantified [6].
FlexMaze assay Mice freely navigated a corridor with staggered doors (8 × 80 cm, 15 min) that required lateral flexion [6].
Radiating pain
Both hindpaws were assessed with a 15-min interval and averaged.
Cold sensitivity The total duration of acetone-evoked behaviours (e.g. flinching, licking or biting) was measured after a drop (25 μL) that was applied to the plantar surface [6].
Mechanical sensitivity von Frey filaments (Stoelting Co., Wood Dale, IL, USA) were applied to the plantar surface using the up-and-down method as previously described [6].
Disc degeneration assessment
Lumbar spine X-ray
Disc height index (DHI) was measured using lateral X-ray images of the spines (Faxitron MX-20 X-Ray LLC, Lincolnshire, IL, USA) performed under deep anaesthesia [4].
Lumbar spine magnetic resonance imaging: preparation, scans, and quantification
After vascular rinse and fixation (4% PFA, intra-cardiac), the T1 to S4 spinal segment was harvested, post-fixed (48 h), embedded (15 mL tube, 10% agarose) and scanned using Bruker standard sagittal RARE-T2 images on a Biospec 70/30 (7 T, Avance III) with Paravision 5.1 (Bruker BioSpin Corporation, Billerica, MA, USA) [3]. Scans were analysed using MIPAV software package, version 7.1.1. using the median section for each lumbar disc. Each region of interest (ROI) included cartilaginous endplates, annulus fibrosus (AF), nucleus pulposus (NP) and extruded material for bulging discs.
Histological assessment
After completion of MRI, samples were decalcified (4% EDTA), cryoprotected, embedded in OCT (Tissue-Tek) and sectioned in the sagittal plane (16 μm) using a cryostat.
Histological staining
FAST staining was performed as previously described [4].
Immunofluorescence histochemistry
Immunohistochemistry was performed using rabbit anti-PGP9.5 (1:1000, Ultraclone, RA95101) and sheep anti-CGRP (1:2000, BML‐CA1137‐0100; Enzo Life Sciences) as previously described [4]. Images were quantified using ImagePro on three non-consecutive sections and averaged for each disc of each animal.
Data analysis
Comparisons between sham and injured mice of each cohort were performed using 2-way ANOVA followed by Fisher’s LSD using Graphpad Prism 8.0 software. Data are expressed as mean ± SEM. All experiments were performed blind to the surgical status.
Results
Time-course of behavioural changes following L4/5-IVD puncture injury
Mice with L4/5-IVD injury had similar (0.5- and 3-months) or increased weight (12 months) compared to their sham controls. The increase at 12 months post-injury was reversed by long-term physical activity (Fig. 1a). While no changes in locomotor capacity were associated with L4/5-IVD injury in the rotarod assay, active animals (sham and injured) had increased capacity compared to age-matched sedentary animals (Fig. 1b). L4/5-IVD injury resulted in a delayed decrease in voluntary wheel running at 12-months that was not observed at earlier time points; this deficit was blocked by physical activity (Fig. 1c). Axial discomfort was assessed using the grip force, tail suspension and FlexMaze assays (Fig. 1d–f and Supplementary Fig. 1). These behavioural assays discriminated between animals with spontaneous or induced IVD degeneration [3, 6,7,8,9], but no persistent differences were observed in the current study. Increased activity had no major impact on axial discomfort, with the exception of the increased grip strength of active injured mice 12 months post-injury (Fig. 1d).
Long-term behavioural consequences of L4/5-IVD injury in active and sedentary mice. 0.5-, 3- or 12-months following L4/5-IVD injury (dark grey) or sham surgery (light grey), cohorts of sedentary and active mice were evaluated for general health (a body weight (in grams), b locomotor capacity (in seconds) in the rotarod assay, c voluntary running capacity assessed as the number of revolutions performed on a wireless Counting Wheels over 1 h), axial discomfort (d Resistive force (in grams) during the grip force assay, e percentage of time in immobility during the 3-min tail suspension assay, f tolerance to lateral bending measured as change in exploration speed in the FlexMaze assay), and radiating discomfort (g cold sensitivity assessed as time spent in acetone-evoked behaviour (sec), h mechanical sensitivity measured as 50% withdrawal threshold (in grams) in the von Very assay). 2-way ANOVA follow by Fisher's LSD; *p < 0.05, **p < 0.01. N = 5–14
Development and maintenance of signs of radiating pain were evaluated. After L4/5-IVD injury, mice demonstrated hyper-sensitivity to cooling stimuli at 3- and 12-month post-injury that was reversed in active mice (Fig. 1g). Consistent with previous studies [3], no changes in the 50% withdrawal threshold in response to mechanical stimuli were observed (Fig. 1h).
Time-course in radiographic (X-ray) and structural (T2-MRI) changes following L4/5-IVD puncture injury
X-ray
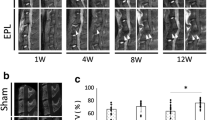
Disc injury resulted in loss of L4/5-IVD height as early as 2 weeks after puncture that persisted throughout the duration of the study. Long-term running activity protected against the loss of disc height 12 months post-injury (Fig. 2b).
Long-term histologic and radiologic consequences of L4/5-IVD injury in active and sedentary mice. a Example of histological (FAST staining), X-ray, and T2-MRI performed in the same sample (also see Fig. 3a, mouse H3). The lumbar spine used here was harvested in a sedentary mouse 12 months after. The injured L4/5-IVD shows signs of degeneration upon histological (collapsed disc with no distinctive nucleus pulposus) and T2-MRI (dark disc) examinations. In this example, the uninjured L2/3-IVD also shows a typical degenerative pattern (i.e. no distinctive nucleus pulposus upon histological analysis, and dark disc upon T2-MRI analysis). # secondary degenerative L2/3-IVD. b–d 0.5-, 3- or 12-months following L4/5-IVD injury (dark grey) or sham surgery (light grey), cohorts of sedentary and active mice were evaluated for anatomic change. b Disc Height Index was measured on X-ray images. Disc hydration (c) and compartmentalization (d) were evaluated on T2-MRI images as median signal intensity and standard deviation for each lumbar IVD. 2-way ANOVA follow by Fisher’s LSD; *p < 0.05, ***p < 0.001. N = 5–14
T2-MRI
The signal intensity median value and standard deviation (SD/signal) were measured on T2-MRI images, and reflect disc hydration and compartmentalization both signs of disc health. In L4/5-IVD, hydration and compartmentalization were significantly decreased 2 weeks post-injury (Fig. 2c, d). While compartmentalization did not evolve further hydration was progressively reduced with age in both injured and non-injured discs. Long-term physical activity had no impact on T2-MRI measures in this study.
Impact of L4/5-IVD puncture injury on adjacent discs
To examine the impact of disc injury on adjacent lumbar discs, the SD of the signal intensity (SD/signal) for each lumbar IVD was determined. The SD/signal ranged from 1111 to 6649. For each cohort, the average (XSD/signal)-1 SDSD/signal of the L4/5-IVD measurements in the sham animals was set as the minimum value for a healthy disc. A SD/signal above this value indicates compartmentalization is intact (healthy), a SD/signal below suggests compartmentalization is compromised (unhealthy) (Fig. 3).
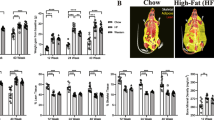
Injured versus secondary degenerating discs in active and sedentary mice. a Heat map of individual lumbar degeneration upon T2-MRI examination, 0.5-, 3- or 12-months following L4/5-IVD injury (dark grey) or sham surgery (light grey) in cohorts of sedentary and active mice. The scale for grey intensity was automatically adjusted for each cohort to avoid age-related bias as follows: X = (averagesham(L4/5-IVD) − 1 SDsham(L4/5-IVD)) for each cohort [X = 3062 (0.5 M-cohort), X = 3720 (3 M-cohort) and X = 4392 (12 M-cohorts)]. b, c total (PGP9.5-ir, Fig. 3b-left panel) and nociceptive (CGRP-ir, b right panel) innervation, and Disc Height Index (c) were measured in sedentary (a− c) or active (d) mice 12-months post-surgery. Measures are presented for L4/5-IVD [sham (light grey) vs. injured (dark grey) mice] or for other level (L1/2, L2/3, L3/4, L5/6) uninjured IVDs from L4/5 injured animals. The other level IVds were futher classified as Normal (textured light grey) versus Degenerative (textured dark grey) based on T2-MRI analysis. For additional details, see Supplementary Method. 2-tailed t-test; *p < 0.05, ***p < 0.001
Using this as a measure of degeneration, we observed an increase in adjacent lumbar disc degeneration with time (Fig. 3a). This was particularly noticeable in the sedentary 12-month post-injury cohort, which presented with numerous degenerating discs in the upper lumbar levels. In the active 12-month injured cohort, the lower lumbar discs were mostly affected (Fig. 3a).
Adjacent discs: innervation and height
In the 12-month post-injury sedentary and active cohorts, lumbar IVDs other than L4/5 were categorised as normal, degenerating or intermediate using the SD of the MRI signal intensity (SD/signal, see suppl. Figure 3). Mechanically injured and uninjured IVDs with secondary degeneration had similar MRI measurements, and no effect of running was observed. In contrast, increased innervation was observed in L4/5 injured IVD but not in the secondary degenerating IVDs (Fig. 3b). Finally, when segregated based on the MRI categories, disc height in non-injured degenerating IVDs was protected by running activity (Fig. 3c).
Discussion
In mice, a single-level L4/5-IVD injury produced robust signs of radiating pain that were alleviated in animals with unlimited access to a home cage activity wheel. By 12-months post-injury, disc degeneration spreads to other levels throughout the lumbar spine. Those secondary degenerating discs presented radiologic signs of degeneration but lacked the pathological innervation observed in the directly injured IVDs. We propose the secondary discs should be further investigated as they may represent a clinically-relevant aspect of human disc degeneration.
Single L4/5-IVD injury and signs of discomfort in mice
Mechanical L4/5-IVD injury did not result in persistent changes in general health or axial discomfort for up to one year following injury. However, consistent with previous studies [3], signs of radiating pain (hyper-sensitivity to hindpaw cooling) were observed and were reversed by long-term physical activity.
Axial discomfort suggestive of discogenic pain
The lack of behavioural signs of axial pain in this study is in conflict with previous report on L4/5-IVD injury-induced pain behaviour [3], where behavioural signs of axial discomfort peaked 3 to 9 months after injury. While it is possible the current study missed the critical time window, it is also clear that assessment of axial discomfort in rodents is a major challenge [10]. This is further amplified by the lack of consensus on disc injury models and the behavioural methods used (i.e. altered physical function [11, 12], rearing [6, 13], grip test or tail suspension [14]) that transfer poorly from one model to another, even within a single team. Since the utility of clinically-relevant animal models of back pain depends on our ability to assess axial discomfort, new creative approaches to behavioural assays are desperately needed [10].
In contrast to the behavioural measures, radiologic (X-ray), structural (MRI) and physiological (innervation) consequences of disc injury are stable and reproducible. This mismatch highlights the poor correlation between the severity of the disease and the behavioural changes observed in animals. Thus, in rodents, as in humans, back pain is not always synonymous with disc degeneration [15].
Hindpaw hyper-sensitivity suggestive of radicular pain
While axial discomfort remains technically challenging to evaluate in animals, most studies investigating behavioural consequences of disc injury/degeneration report hyper-sensitivity to stimuli applied to hindpaws. In our initial publication [3], we observed that cold hyper-sensitivity developed in a biphasic fashion with an early, transient peak at 0.5-months post-injury that resolved by 6-months and re-emerged at 12-months. Here we observed a delay in the onset of cold hyper-sensitivity at 3- and 12- but not 0.5-months post-injury.
In contrast to the delayed onset of radicular pain, the severity of degeneration of the injured discs by both X-ray and MRI was fully established by 0.5-months and persisted throughout the study, suggesting a mismatch between radiating pain and degeneration severity. In primary care, approximately 60% of patients with low back pain also report pain in one or both legs [16]. Interpedicular height has been suggested as a predictor of radicular pain in adult degenerative scoliosis [17] and mechanical radiating pain co-varies with severity in disc narrowing in a rat model of disc injury [18]. In our previous studies with both induced and progressive models of mouse IVD degeneration, disc height has been consistently associated with cold hyper-sensitivity on the hindpaw [3, 6, 7].
In the current study, long-term access to voluntary running wheels was used to model an active lifestyle and prevented signs of radiating pain 12-months post-injury. Activity also attenuated the loss of disc height in secondary degenerating IVDs, furthering the argument for a direct relationship between pathologic disc narrowing and radiating discomfort.
Progression of disc degeneration
We performed radiologic and histological analysis of the 5 lumbar discs for each animal, including both injured and uninjured L4/5 and adjacent segments of the spine.
Progression of disc degeneration over time
We previously reported increased innervation and severe degeneration by histological assessment in injured IVDs as early as 0.5 M post-injury that persisted for up to one year [4]. In the current study, we demonstrate that degeneration severity of injured L4/5-IVDs was similar from 0.5 to 12 months by radiologic (X-ray and MRI) measurements. These data suggest that structural damage to the injured IVD does not evolve further after the acute injury. Long-term physical activity had no impact on radiologic measures of the injured IVD. However, since non-injured IVDs narrow with age, the differences between injured and non-injured IVDs faded with time.
Progression of degeneration in adjacent segments over time
A major finding of this study is that disc degeneration spreads to adjacent discs with time in mice. The secondary degenerating discs had reduced height and altered MRI signal of similar magnitude to the primary injured disc; radiologically, it would be difficult to distinguish between mechanically injured and non-injured secondary degenerating IVDs 12-months post-injury. In contrast, these secondary degenerating discs were not abnormally innervated. Long-term physical activity prevented the secondary degenerating discs from narrowing but had no impact on MRI signal intensity.
The adjacent segment spread is clinically-relevant and is well documented in humans both spontaneously and after spinal fusion [19,20,21] or vertebral fracture [22]. It has also been described after spinal fusion in animals such as goats [23] or ovariectomized rats [24]. To our knowledge, this is the first time that the degeneration of discs other—and sometimes even distant—than the mechanically damaged one has been documented, without spinal fusion, in mice. This clearly calls into question the validity of using adjacent discs as controls in molecular, physiological or biomechanical studies in animals.
In this model, the spreading of degeneration cannot be attributed to genetic predisposition or congenital weakness. Rather, it is a downstream consequence of the initial L4/5 injury, perhaps related to spinal instability. Additional studies of these secondary degenerating discs are needed, including assessment of sympathetic innervation, vascularization, pro-inflammatory and pro-nociceptive factors (e.g. TNFa, IL8, NGF) and extracellular matrix remodelling. We suggest that secondary degenerating discs represent a clinically-relevant aspect of degeneration of human discs, especially in cases where multiple levels are affected.
Conclusions
Here we report the spread of degeneration to other lumbar discs following L4/5-IVD injury. The secondary degenerating discs lacked the pathological innervation observed in the directly injured IVDs. Increased physical activity attenuated behavioural signs of radiating pain and prevented the loss of disc height in non-injured, secondary degenerating discs. These studies support physical activity-based interventions for discogenic low back pain and call for additional focus on adjacent segment degeneration, which may represent a clinically-relevant model of the human condition.
Availability of data and material
Available upon request.
References
Global Burden Of Disease Study C (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet 386:743–800. https://doi.org/10.1016/S0140-6736(15)60692-4
Ja H, Mw VT, Av M, Bw K (2005) Meta-analysis: exercise therapy for nonspecific low back Pain. Ann Intern Med 142:765–775. https://doi.org/10.7326/0003-4819-142-9-200505030-00013
Millecamps M, Ls S (2018) Delayed onset of persistent discogenic axial and radiating pain after a single-level lumbar intervertebral disc injury in mice. Pain 159:1843–1855. https://doi.org/10.1097/J.Pain.0000000000001284
Lee S, Millecamps M, Dz F, Ls S (2020) Long-term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury-induced low back pain. J Orthop Res 38:1238–1247. https://doi.org/10.1002/Jor.24560
Zimmermann M (1986) Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand Suppl 554:221–233
Millecamps M, Tajerian M, Naso L, Sage Eh, Ls S (2012) Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing sparc-null mice. Pain 153:1167–1179. https://doi.org/10.1016/J.Pain.2012.01.027
Millecamps M, Jt C, Ap M, Ls S (2015) Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J 15:2524–2537. https://doi.org/10.1016/J.Spinee.2015.08.055
Miyagi M, Millecamps M, Danco At, Ohtori S, Takahashi K, Stone Ls (2014) Issls prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 39:1345–1354. https://doi.org/10.1097/Brs.0000000000000334
Tajerian M, Millecamps M, Ls S (2012) Morphine and clonidine synergize to ameliorate low back pain in mice. Pain Res Treat 2012:150842. https://doi.org/10.1155/2012/150842
Shi C, Qiu S, Sm R, Das V, Zhu B, Aa W, Aj VW, Mwale F, Jc I, Sakai D, Votta-Velis G, Yuan W, Hj Im (2018) Animal models for studying the etiology and treatment of low back pain. J Orthop Res 36:1305–1312. https://doi.org/10.1002/Jor.23741
Olmarker K (2008) Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine (Phila Pa 1976) 33:850–855. https://doi.org/10.1097/Brs.0b013e31816b46ca
Allen Kd, Tm G, Rm R, Wc W, Vb K, Jl H, Lm B, La S (2009) Decreased physical function and increased pain sensitivity in mice deficient for type Ix collagen. Arthritis Rheum 60:2684–2693. https://doi.org/10.1002/Art.24783
Shi C, Das V, Li X, Kc R, Qiu S, Os I, Rl R, Js K, Mwale F, Aa W, Zhu B, Zhao L, Aj VW, Ji M, Lu J, Votta-Velis G, Yuan W, Hj Im (2018) Development of an in vivo mouse model of discogenic low back pain. J Cell Physiol 233:6589–6602. https://doi.org/10.1002/Jcp.26280
Millecamps M, Tajerian M, Sage Eh, Stone Ls (2011) Behavioral signs of chronic back pain in the sparc-null mouse. Spine (Phila Pa 1976) 36:95–102. https://doi.org/10.1097/Brs.0b013e3181cd9d75
Brinjikji W, Fe D, Jg J, Cm C, Df K, Murad Mh, Luetmer Ph (2015) Mri findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. Ajnr Am J Neuroradiol 36:2394–2399. https://doi.org/10.3174/Ajnr.A4498
Konstantinou K, Dunn Km, Ogollah R, Vogel S, Hay Em, Team Asr (2015) Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the Atlas cohort study. BMC Musculoskelet Disord 16:332. https://doi.org/10.1186/S12891-015-0787-8
Hawasli Ah, Chang J, Ck Y, Steger-May K, Lg L, Ig D (2016) Interpedicular height as a predictor of radicular pain in adult degenerative scoliosis. Spine J 16:1070–1078. https://doi.org/10.1016/J.Spinee.2016.04.017
Lai A, Ho L, Tw E-R, Watanabe H, Salandra J, Ba W, Laudier D, Ac H, Gm P, Jc I (2019) Dietary polyphenols as a safe and novel intervention for modulating pain associated with intervertebral disc degeneration in an in-vivo rat model. PLoS ONE 14:E0223435. https://doi.org/10.1371/Journal.Pone.0223435
Aa G (2016) Adjacent segment degeneration after posterior lumbar fusion: an analysis of possible risk factors. Clin Neurol Neurosurg 143:15–18. https://doi.org/10.1016/J.Clineuro.2016.02.004
Fm S-P, Ra D, Ec B (2014) Adjacent segment disease perspective and review of the literature. Ochsner J 14:78–83
As H, Robbins M (2004) Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 4:190s–194s. https://doi.org/10.1016/J.Spinee.2004.07.007
Dc N, Marcia S, Ardura F, Is L, Marras M, Saba L (2016) Diffusion-weighted Mri assessment of adjacent disc degeneration after thoracolumbar vertebral fractures. Cardiovasc Intervent Radiol 39:1306–1314. https://doi.org/10.1007/S00270-016-1369-3
Hoogendoorn Rj, Helder Mn, Wuisman Pi, Bank Ra, Everts Ve, Smit Th (2008) Adjacent segment degeneration: observations in a goat spinal fusion study. Spine (Phila Pa 1976) 33:1337–1343. https://doi.org/10.1097/Brs.0b013e318173438f
Cc L, Fm T, Zhou Z, Wang P, Gou Y, Zhang H, Wy W, Shen Y, Yz Z, Zhang L (2015) Protective effect of calcitonin on lumbar fusion-induced adjacent-segment disc degeneration in ovariectomized rat. BMC Musculoskelet Disord 16:342. https://doi.org/10.1186/S12891-015-0788-7
Acknowledgements
The authors thank the staff of McGill University’s Comparative Medicine and Animal Resources Centre, Alan Edwards Centre for Research on Pain, Douglas Brain Imaging Centre, and Centre for Bone and Periodontal Research for their support and expertise.
Funding
This work was supported by Canadian Institutes for Health Research Grants MOP-126046 and MOP-142291 to LSS and MM. SL was supported by the Catherine Bushnell postdoctoral fellowship from the Louise and Alan Edwards Foundation.
Author information
Authors and Affiliations
Contributions
Data collection MM, PL, DF; Methodology, Material preparation, Project administration, Formal analysis: MM; Funding acquisition and Conceptualization by MM and LSS, Supervision and manuscript review & editing: LSS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Millecamps, M., Lee, S., Foster, D.Z. et al. Disc degeneration spreads: long-term behavioural, histologic and radiologic consequences of a single-level disc injury in active and sedentary mice. Eur Spine J 30, 2238–2246 (2021). https://doi.org/10.1007/s00586-021-06893-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-06893-2