Abstract
Purpose
Although lung volume (LV) can be measured directly by computed tomography (CT), the literature regarding CT-assessed LV in adolescent idiopathic scoliosis (AIS) patients is limited, and the influence of posterior spinal fusion with instrumentation (PSF) on LV has not been established. This study aimed to identify factors associated with decreased LV after PSF in AIS patients.
Methods
We retrospectively reviewed 111 consecutive AIS patients who were between 10 and 20 years of age and were treated by PSF at our facility. We assessed age at surgery, sex, height, body weight, Risser stage, Lenke classification, radiographic parameters, pulmonary function tests, and LV. Factors associated with a postoperative decrease in LV were identified by multivariable analysis.
Results
The mean total LV had increased at the 2-year follow-up, although marginally significant (p = .06), and there was a significant increase in the left LV (p = .01) but not the right LV (p = .25). We observed a postoperative reduction in total LV, defined as a total LV postoperative/preoperative ratio < 0.9, in 20 of the 111 patients (18.0%). Univariable analysis showed a significant correlation between ≥ 11 fusion levels and postoperative LV reduction (OR 3.11, 95% CI 1.13–8.57). This factor remained significant in the multivariable analysis, which yielded an adjusted OR of 2.82 (95% CI 1.01–7.93) for postoperative LV reduction in patients with ≥ 11 fusion levels.
Conclusion
Our data suggest that a longer fusion area is associated with postoperative LV reduction. Therefore, avoidance of a longer fusion area of ≥ 11 will be preferable for preserving LV.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired pulmonary function is a common complication of adolescent idiopathic scoliosis (AIS), due to deformity of the spine and ribcage [1, 2]. Several factors that can potentially affect pulmonary function in patients with AIS have been identified, including structural features, the proximal thoracic curve, apex displacement, sagittal hypokyphosis and even thoracic lordosis, and the number of vertebrae involved in the major curve [2, 3]. One of purposes of corrective surgery for AIS is to prevent the deterioration in pulmonary function caused by progressive spinal deformity. Particularly, posterior spinal fusion with instrumentation (PSF) has been shown to yield small or moderate increases in pulmonary function in AIS patients [4,5,6]. However, the underlying mechanisms of pulmonary impairment in AIS patients, and of its postoperative improvement, is not fully understood.
Pulmonary function testing (PFT) is commonly used to evaluate the effect of obstructive and restrictive pulmonary diseases on respiratory function [7, 8]. However, AIS is often initially assessed in children who are too young to cooperate with the PFT protocol [9]. Computed tomography (CT) can accurately measure actual lung volume (LV) in this population [3, 10,11,12,13,14].
Although studies have reported that corrective surgery for AIS improves pulmonary function, two studies found no significant postoperative improvements in total LV among patients with AIS who underwent PSF [11, 13], and Lee et al. reported a significant decline in total LV at 4 weeks post-PSF in this population [12]. However, given the relatively small number of subjects and short follow-up periods in these studies, they might not represent the ultimate outcomes of these patients.
Between 2007 and 2014, patients who were treated surgically for AIS at our institution underwent low-dose CT scans prior to surgery, to develop the surgical strategy, and 2 years after surgery to detect postoperative screw loosening and perforation especially in the upper and lower instrumented vertebrae [15]. Thus, we were able to review detailed LV data for a relatively large pool of AIS patients treated by PSF. For this analysis, we used an established technique for reconstructing pulmonary anatomy from low-dose CT scans [10, 16]. We first analyzed differences between the preoperative and postoperative LV in the AIS patients in this dataset. We then sought to identify the factors associated with a postoperative LV reduction in this population.
Methods
Subjects
We retrospectively reviewed the data for consecutive AIS patients (aged 10–20 years) who had right-side major thoracic curves and were treated by PSF at our institution between 2007 and 2014. All patients had complete radiography, CT, and PFT data from the preoperative assessment and the 2-year follow-up. After excluding patients with a Lenke type 5 curve, 111 patients (103 female, 8 male) were included in the study. The following data were collected and analyzed: age, sex, height, body weight, Risser stage, Lenke classification, radiographic parameters, PFT data, and LV values measured on reconstructed CT. The mean age, height, body weight, and body mass index are shown in Table 1. By Lenke curve type, there were 66 type 1 patients, 20 type 2, 7 type 3, 4 type 4, and 14 type 6. By Risser stage, there were 8 stage 0 patients, 5 stage 1, 16 stage 2, 15 stage 3, 44 stage 4, and 23 stage 5 (Table 1). This research was approved by our institution’s review board. We obtained written informed consent for the use of patient data from each patient or their guardian, according to the hospital’s ethics guidelines.
Surgical procedure
All surgeries were performed by the same experienced surgical team and under general anesthesia and neuro-monitoring. Extent of fusion was basically chosen according to Lenke classification. The patient was positioned prone on a spinal frame. A cell saver was used routinely. A medial skin incision was used to expose the posterior elements. A bilateral partial facetectomy was performed at each level. Pedicle screws were inserted bilaterally at all levels using a free hand technique, except for some cases (n = 30, 27.0%) in which pedicle width was too narrow for screw insertion. A gradual correction was done under neuro-monitoring supervision guidance. A wide decortication and spread of autologous bone graft was carried out.
Radiographic parameters
We used full-length standing posteroanterior and lateral radiographs of the spine, obtained before surgery and 2 years after surgery. The following radiographic parameters were evaluated: magnitude of the proximal thoracic, main thoracic, and thoracolumbar/lumbar curves; upper thoracic (T2–T5) and main thoracic kyphosis (T5–T12); lumbar lordosis (T12 to sacrum); sagittal vertical axis (SVA); apical vertebral translation, defined as the distance between the apex of the main thoracic curve and the C7 plumb line (C7PL); and coronal balance, defined as the distance between the center sacral vertical line (CSVL) and the C7PL. The coronal balance was defined as negative when the C7PL was to the left side of the CSVL.
Pulmonary function testing
We measured the following parameters by PFT before the operation and at the 2-year follow-up: vital capacity (VC), the maximum volume of air that can be exhaled after a maximum inspiration; the forced expiratory volume in 1 s (FEV1), the amount of air expired in 1 s during a rapid and maximal expiration from full inspiration; and FEV 1% which represents the FEV1/ forced VC ratio. For calculating %VC, the value of VC was normalized to the patient’s age, sex, height, or arm span-matched standards, according to the Japanese Respiratory Society guidelines [17].
Computed tomography scanning and quantitative image analysis
The preoperative and 2-year postoperative CT examinations were obtained with a 16-, 64-, or 320-detector CT scanner. Low-dose CT scans were acquired at 0.5-mm or 0.625-mm collimation with autoexposure control (depending on the size of the patient) to generate contiguous 1-mm-thick or 1.25-mm-thick CT images. The effective dose estimate for CT was 4.1 ± 0.9 mSv, which was determined by the dose length product measurements and appropriate normalized coefficients reported in the literature [18]. LV values were measured on preoperative and 2-year postoperative CT images by a board-certified radiologist with 15 years of experience, using a commercially available workstation (Advantage Workstation 4.6, GE Healthcare, Waukesha, WI, USA) (Fig. 1). We measured the right lung volume (RLV), left lung volume (LLV), and total lung volume (TLV) and calculated the ratio of the right lung to left lung volume (RLV/LLV ratio). We calculated the ratio of the postoperative to preoperative TLV volumes for each patient, and defined a ratio less than 0.9 as a postoperative LV reduction.
Statistical analysis
Data are presented as the mean ± standard deviation. Differences between preoperative and postoperative variables were evaluated for significance by paired t test. We calculated Pearson’s correlation coefficients to evaluate correlations between TLV and PFT variales including VC and FEV1. We applied a logistic regression model to examine associations between individual risk factors and postoperative LV reduction and estimated the odds ratios (ORs) and 95% confidence intervals (95% CIs) for postoperative LV reduction. Logistic regression was performed with STATA14 software (Stata Corporation, College Station, TX, USA). A p value less than 0.05 was considered statistically significant.
Results
Preoperative and postoperative radiographic parameters, PFT data, and LV Table 2 shows the preoperative and 2-year postoperative radiographic parameters and PFT data used in this evaluation. From the preoperative assessment to the 2-year follow-up, the average Cobb angle decreased significantly for the proximal thoracic (27.8° ± 9.3° vs 13.9° ± 6.6°; p < 0.01), main thoracic (54.9° ± 10.4° vs 17.4° ± 7.5°; p < 0.01), and thoracolumbar/lumbar curves (34.0° ± 13.4° vs 10.2° ± 7.9°; p < 0.01). We also observed significant differences between the preoperative and 2-year postoperative coronal balance (0.6 ± 16.8 mm vs − 4.6 ± 10.7 mm, p = 0.01) and apical translation (43.8 ± 37.8 mm vs − 2.6 ± 18.6 mm; p < 0.01). For sagittal radiographic parameters, we observed an increase, although not significant, in the magnitude of upper thoracic kyphosis (T2–T5; p = 0.07) and main thoracic kyphosis (T5–T12; p = 0.10) at the 2-year follow-up. Meanwhile, the average value of SVA was significantly decreased from the preoperative assessment to the 2-year follow-up ( − 16.9 ± 22.4 mm vs − 25.9 ± 21.0 mm; p < 0.01). PFT showed a significant decrease in %VC (p < 0.01) and significant increases in VC (p = 0.02), FEV1 (p < 0.01), and FEV 1% (p < 0.01) at the 2-year follow-up. Table 3 shows the preoperative and postoperative LV values. TLV increased from the preoperative evaluation (2.75 ± 0.65 L) to the 2-year follow-up (2.82 ± 0.65 L); this increase was marginally significant (p = 0.06). LLV increased significantly from the preoperative (1.24 ± 0.31 L) to the postoperative evaluation (1.29 ± 0.32 L; p = 0.01); an increase in RLV (from 1.51 ± 0.36 L to 1.53 ± 0.34 L) was not significant (p = 0.25). The RLV/LLV ratio decreased significantly from the preoperative (1.22 ± 0.15) to the 2-year postoperative evaluation (1.20 ± 0.11; p = 0.02).
Correlations between PFT data and TLV
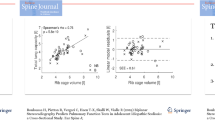
To validate TLV as an indicator of pulmonary function, we analyzed correlations between TLV and the pulmonary function variables including VC and FEV1 at pre- and post-operation. Notably, we found that TLV was correlated moderately or strongly with VC and FEV1 at both pre- and post-operation (r = 0.66 – 0.92, p < 0.01) (Fig. 2a, b). In addition, the TLV postoperative/preoperative ratio was correlated weakly but significantly with those ratios for both the VC (r = 0.34, p < 0.01) and the FEV1 (r = 0.35, p < 0.01) (Fig. 2c).
Analysis of factors associated with postoperative LV reduction
Of the 111 patients, 20 (18.0%) had a postoperative LV reduction (Table 4). We used a logistic regression model to evaluate associations between postoperative LV reduction and age, sex, Risser sign, Lenke classification, number of fusion levels, level of the upper instrumented vertebra (UIV), preoperative magnitude of the main thoracic curve, operative change in thoracic kyphosis, and correction rate of the Cobb angle of the main thoracic curve. Univariable analysis revealed an association between postoperative LV reduction and a fusion length of ≥ 11 levels (OR 3.11, 95% CI 1.13–8.57). After adjusting for age (p value in univariable analysis = 0.44) and the magnitude of the operative main thoracic curve prior to surgery (p value in univariable analysis = 0.15), this association was still significant (OR 2.82, 95% CI 1.01–7.93).
Preoperative and postoperative PFT data in cases with fusion length of 6–10 levels and 11–15 levels
Preoperative and postoperative PFT data were separately assessed in cases with fusion length of 6–10 levels and 11–15 levels (Table 5). The value of VC, FEV1, and FEV1% was significantly increased from the preoperative to the postoperative evaluation in cases with fusion length of both 6–10 levels and 11–15 levels (Table 5). Notably, the value of %VC in cases with fusion length of 11–15 levels significantly decreased from the preoperative assessment to the 2-year follow-up, whereas that of 6–10 levels was comparable between preoperatively and postoperatively (Table 5). These observations supported our multivariable analysis results.
Discussion
Several studies have analyzed PFT data in patients with AIS. However, few have used CT images to measure and analyze preoperative and postoperative LV values [11, 13, 19]. To the best of our knowledge, this retrospective study is the largest to evaluate LV values after PSF in a population of patients with AIS (n = 111) who were followed at least 2 years [11, 13]. At the 2-year follow-up, TLV had increased, although marginally significant. Importantly, there was a significant increase in LLV but not RLV. In addition, our study is the first to examine risk factors for postoperative TLV reduction. Although our findings should be validated with a larger sample, the present study indicates that a longer fusion length ( ≥ 11 levels) may be a risk factor for a postoperative reduction in TLV.
Our results demonstrated significant associations between TLV and PFT data, including VC and FEV1, verifying TLV as an indicator of pulmonary function in patients with AIS. Whereas PFT evaluates dynamic breathing parameters involving the movement of the chest and rib cage, such as deep expiration and inspiration, LV calculated by reconstructing CT scans represents the static component of the lungs. The CT scan method is particularly useful for measuring LV accurately in patients who are unable to follow instructions for PFT [3, 10,11,12,13,14], and it has the added advantage of measuring the right and left sides separately. CT-based LV measurements can provide further our understanding of anatomical characteristics associated with scoliosis and marked left–right asymmetry. Sarwahi et al. [13] assessed LV in 29 AIS patients and reported that some LV values were not altered after PSF. Similarly, our study indicated that PSF did not significantly increase the TLV. However, a more detailed analysis showed a significant postoperative increase in the LV at the concave side of the major curve, but not at the convex side. One possible explanation for this observation is that vertebral de-rotation for correction expands the LV at the concave side and contracts that of the convex side.
Karol et al. [20] reported that the proximal extent of the fusion increases the risk of developing restrictive pulmonary disease in patients younger than 9 years who were treated by thoracic spine fusion for non-neuromuscular scoliosis. In contrast, we did not find a significant correlation between UIV level and postoperative LV reduction in AIS patients. Demura et al. [21] reported that the presence of an upper thoracic curve in AIS was associated with decreased PFT values at the 2-year follow-up. However, in the present study, the presence of an upper thoracic curve (Lenke type 2) was not significantly associated with a postoperative LV reduction. Although these discrepancies between studies might be due to differences in object variables and analysis methods, these issues deserve further investigation. In the present study, univariable logistic regression analysis identified a fusion length of ≥ 11 vertebrae as a potential factor in postoperative LV reduction. Because cases with a construct ≥ 11 levels have more severe deformity compared with those with a construct < 11 levels (Supplementary Table 1), multivariable analysis was conducted by adjusting the factors including preoperative Cobb angle of main thoracic curves. Breathing is partially affected by spinal movement [22], which becomes more restricted as the fusion length increases, and this condition might be a factor in reducing the postoperative LV in AIS patients. Thus, our results suggest that using as short a fusion area as possible may help maintain LV after PSF.
This study had several limitations. This was a single-center study, with potential selection bias. Second limitation was a lack of direct comparison with anticipated changes in LV of a normal age-matched population or of a matched AIS group that did not have PSF. The normative reference data of Gollogly et al. [16] can be used for comparison. However, their study lacks the data of AIS patients aged 10–20 years. Without benchmarks, it is difficult to assess exactly whether PSF had any impact on LV. Third, despite being the largest study of LV values after PSF [11,12,13], the sample size might not be large enough to draw solid conclusions from our findings. Finally, we do not recommend the routine use of CT to assess LV due to concerns about increasing the radiation burden, especially given recent reports of risks of induced malignancy [23]. Nevertheless, the present study successfully demonstrated the postoperative change in LV values after PSF in a relatively large subject sample.
In conclusion, we retrospectively analyzed LV prior to and 2 years after PSF in patients with AIS and conducted the first-ever multivariable analysis of risk factors for postoperative LV reduction. Our results indicate that a longer fusion area may lead to a postoperative LV reduction. Therefore, avoidance of a longer fusion area of ≥ 11 will be preferable for preserving lung volume.
Abbreviations
- AIS:
-
Adolescent idiopathic scoliosis
- PSF:
-
Posterior spinal fusion with instrumentation
- PFT:
-
Pulmonary function testing
- CT:
-
Computed tomography
- LV:
-
Lung volume
- CSVL:
-
Center sacral vertical line
- C7PL:
-
C7 plumb line
- VC:
-
Vital capacity
- FEV1:
-
Forced expiratory volume in 1 s
- RLV:
-
Right lung volume
- LLV:
-
Left lung volume
- TLV:
-
Total lung volume
- RLV/LLV ratio:
-
Ratio of the right lung to left lung volume
- UIV:
-
Upper instrumented vertebra
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
References
Chu WC, Li AM, Ng BK, Chan DF, Lam TP, Lam WW, Cheng JC (2006) Dynamic magnetic resonance imaging in assessing lung volumes, chest wall, and diaphragm motions in adolescent idiopathic scoliosis versus normal controls. Spine 31(19):2243–2249
Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG (2005) Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty-one patients. J Bone Joint Surg Am 87 (9):1937–1946
Wen Y, Kai S, Yong-Gang Z, Guo-Quan Z, Tian-Xiang D (2016) Relationship between lung volume and pulmonary function in patients with adolescent idiopathic scoliosis: Computed tomographic-based three-dimensional volumetric reconstruction of lung parenchyma. Clin Spine Surg 29(8):E396
Kumano K, Tsuyama N (1982) Pulmonary function before and after surgical correction of scoliosis. J Bone Joint Surg Am 64(2):242–248
Gagnon S, Jodoin A, Martin R (1989) Pulmonary function test study and after spinal fusion in young idiopathic scoliosis. Spine 14(5):486–490
Lee AC, Feger MA, Singla A, Abel MF, (2016) Effect of Surgical Approach on Pulmonary Function in Adolescent Idiopathic Scoliosis Patients: A Systemic Review and Meta-analysis. Spine 41(22):E1343–E1355
Eisenberg JD, Wall MA (1987) Pulmonary function testing in children. Clin Chest Med 8(4):661–667
Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS (2000) Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med 161 (2 Pt 1):353–9.
Enright PL, Linn WS, Avol EL, Margolis HG, Gong H Jr, Peters JM (2000) Quality of spirometry test performance in children and adolescents : experience in a large field study. Chest 118(3):665–671
Adam CJ, Cargill SC, Askin GN (2007) Computed tomographic-based volumetric reconstruction of the pulmonary system in scoliosis: trends in lung volume and lung volume asymmetry with spinal curve severity. J Pediatr Orthop 27(6):677–681
Fu J, Liu C, Zhang YG, Zheng GQ, Zhang GY, Song K, Tang XY, Wang Y (2015) Three-dimensional computed tomography for assessing lung morphology in adolescent idiopathic scoliosis following posterior spinal fusion surgery. Orthop Surg 7(1):43–49
Lee DK, Chun EM, Suh SW, Yang JH, Shim SS (2014) Evaluation of postoperative change in lung volume in adolescent idiopathic scoliosis: measured by computed tomography. Indian J Orthop 48(4):360–365
Sarwahi V, Sugarman EP, Wollowick AL, Amaral TD, Harmon ED, Thornhill B (2014) Scoliosis surgery in patients with adolescent idiopathic scoliosis does not alter lung volume: a 3-dimensional computed tomography-based study. Spine 39(6):E399–405
Yu CG, Grant CA, Izatt MT, Labrom RD, Askin GN, Adam CJ, Little JP (2017) Change in Lung Volume Following Thoracoscopic Anterior Spinal Fusion Surgery: A 3-Dimensional Computed Tomography Investigation. Spine 42(12):909–916
Uehara M, Takahashi J, Ikegami S, Kuraishi S, Shimizu M, Futatsugi T, Oba H, Koseki M, Kato H (2017) Pedicle Screw Loosening After Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis in Upper and Lower Instrumented Vertebrae Having Major Perforation. Spine 42(24):1895–1900
Gollogly S, Smith JT, White SK, Firth S, White K (2004) The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system. Spine 29(18):2061–2066
Kubota M, Kobayashi H, Quanjer PH, Omori H, Tatsumi K, Kanazawa M; Clinical Pulmonary Functions Committee of the Japanese Respiratory Society (2014) Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 52(4):242–250
Diagnostic Imaging Council CT Committee (2008) The measurement, reporting, and management of radiation dose in CT. American Association of Physicists in Medicine. https://www.aapm.org/pubs/reports/rpt_96.pdf. Accessed April 19, 2019.
Aaro S, Dahlborn M (1981) Estimation of vertebral rotation and the spinal and rib cage deformity in scoliosis by computer tomography. Spine 6(5):460–467
Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH (2008) Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am 90(6):1272–1281
Demura S, Bastrom TP, Schlechter J, Yaszay B, Newton PO; Harms Study Group (2013) Should postoperative pulmonary function be a criterion that affects upper instrumented vertebra selection in adolescent idiopathic scoliosis surgery? Spine 38(22):1920–1926
Leong JC, Lu WW, Luk KD, Karlberg EM (1999) Kinematics of the chest cage and spine during breathing in healthy individuals and in patients with adolescent idiopathic scoliosis. Spine 24(13):1310–1315
Brenner DJ, Hall EJ (2007) Computed tomography-an increasing source of radiation exposure. N Engl J Med 357(22):2277–2284
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fujita, N., Yagi, M., Michikawa, T. et al. Impact of fusion for adolescent idiopathic scoliosis on lung volume measured with computed tomography. Eur Spine J 28, 2034–2041 (2019). https://doi.org/10.1007/s00586-019-06025-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06025-x