Abstract
Purpose
Controlled trials have shown that total disc replacement (TDR) can provide pain and disability relief to patients with degenerative disc disease; however, whether these outcomes can also be achieved for patients treated in normal surgical practice has not been well documented.
Methods
This prospective, international study observed changes in disability and back pain in 134 patients who were implanted with Maverick TDR within the framework of routine clinical practice and followed for 2 years post-surgery. Primary and secondary outcomes were the differences from baseline to 6 months post-surgery in the means of the Oswestry Disability Index and the change in back pain intensity assessed on a 10-cm visual analogue scale, respectively. Mean patient age at surgery was 43 years, but ranged up to 65 years.
Results
One hundred twenty-three patients had an implant at one level, 10 patients at two levels, and one patient at three levels. Statistically significant improvements in mean disability (−25.4) and low back pain intensity (−4.0) scores were observed at 6 months postoperatively (P < 0.0001 for both) in the hands of experienced surgeons (>10 TDRs per centre). During the study, 56 patients (42 %) experienced a complication or adverse event.
Conclusions
This is the first international observational study to report outcomes of TDR in real-world clinical settings. We showed statistically significant improvements in disability and pain scores at 6 months following Maverick TDR, which were maintained for 2 years alongside an acceptable rate of perioperative complications. The safety and tolerability shown in this observational study were comparable to those from controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 50 years, a pathologic disc associated with lumbar degenerative disc disease (DDD) that did not respond to conservative care has been preferentially treated by spinal fusion. As an alternative, in 1984, lumbar spinal arthroplasty or disc replacement emerged [1–4]. Disc replacement can provide pain relief by resecting the diseased intervertebral disc and dynamically stabilising the segment, allowing restoration and maintenance of spine biomechanics. Compared to fusion, this is expected to reduce the incidence of adjacent segment degeneration [5–7].
The Maverick disc, a two-piece lumbar disc prosthesis, can be implanted at any spinal level from T12/L1 to L5/S1. It has a semi-constrained metal-on-metal design, preserving motion by a ball-and-socket construct with a physiological posterior centre of rotation [8]. A 2-year randomised controlled trial (RCT) demonstrated statistical superiority of Maverick over spinal fusion based on key clinical outcomes, including disability and pain [9].
The optimal patient population for total disc replacement (TDR) is not fully defined [9–11]. Available information outside a controlled clinical study setting comes from a single national registry [10, 12]. The objective of this first international observational study was to investigate changes in disability and back pain in a broad patient population treated with Maverick TDR in varied surgical practices reflecting different national standards of care.
Patients and methods
Study design and participants
This prospective observational study of normal surgical practice for the MAV Motion Segment Replacement (MSR; A-MAV and O-MAV; Medtronic Sofamor Danek, Memphis, TN, USA) was conducted at 11 centres in France (7: Clinique de Neurochirurgie Hôpital Roger Salengro, Centre Hospitalier Universitair Pellegrin Tripode; Centre Hospitalier de Meulan, Service de Neurochirurgie Hôp Nice, AP-HP Hôpital Beaujon, Clinique du Cours Dillon, Centre Hospitalier La Timone), Germany (3: Universitätklinikum Magdeburg, Praxis für Orthopädie und Neurochirurgie Potsdam, Charité Berlin–Klinik für Orthopädie), and Canada (1: Montreal General Hospital) from February 2009 to July 2013. One hundred thirty-four patients with back pain were implanted with Maverick discs and followed for 2 years. Study centres were required to have at least 10 TDR per year prior to participation. The study is registered at http://www.clinicaltrials.gov (NCT01338493).
Patients eligible for MAV MSR disc replacement according to the labelling were included at the discretion of the surgeon and managed in routine clinical practice. Patients were not excluded for having fusion-treated degenerative spondylolisthesis adjacent to the implantation level. Patients were enroled by signing informed consent and having their data entered in the electronic case report form (eCRF).
Ethical considerations
This study was conducted in agreement with the Declaration of Helsinki and local regulations of the participating countries. According to the requirements, a notification letter regarding the registry was sent to, or written approval was obtained from, the Ethics Committee (EC)/Institutional Review Board (IRB)/Human Research Ethics Committee (HREC) before the start of the study. Data were collected by web-based eCRF according to the legislation for each participating country. All enrolled patients gave written informed consent in their local language before participation.
Study procedures
Patients were treated by lumbar spinal arthroplasty. A complete anterior discectomy was performed followed by A-MAV (implant for anterior insertion at levels T12–S1, 126 implants) or O-MAV (implant for oblique insertion at levels L4–L5, 20 implants) insertion to replace the damaged lumbar intervertebral disc [8] at up to three levels; patients could also be treated with adjacent fusion. All procedures and assessments (Table 1) were part of the standard treatment a patient outside of the study would also receive.
Outcome measures
Primary outcome was change from baseline at 6 months post-surgery of mean Oswestry Disability Index (ODI version 2.1). Secondary outcome was change at 6 months versus baseline in back pain intensity assessed on a 10-cm visual analogue scale [VAS; 0 cm (no pain) to 10 cm (worst possible pain)]. The percentage of patients attaining a ≥15-point improvement in ODI—criterion for success defined by the US Food and Drug Administration (FDA) [9]—was calculated.
Tertiary outcomes included changes from baseline at 12 and 24 months post-surgery in ODI, leg pain intensity (VAS), back and leg pain frequency [VAS; 0 cm (pain none of the time] to 10 cm (pain all of the time)], and 6 and 24 month patient quality of life improvement from baseline [the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Short-Form Health Survey (SF-36) v2]. The percentage of patients attaining a minimal clinically important difference of a ≥4.9-point increase in PCS [13] was calculated. Baseline and postoperative (6, 12, and 24 months) measures of range of motion at the operated level(s), work status, pain medication, and non-drug pain treatment were also compared. Patient treatment satisfaction and adverse events (AEs) were documented up to 24 months after surgery.
All adverse findings and complications were reported regardless of severity, causation, or relatedness to the implant or surgical procedure. Relationship of AEs to surgery or device was not categorised by the investigators.
Statistical analysis
Statistical analysis was carried out for all patients implanted with a Maverick disc using the Statistical Analysis System (SAS) software package version 9.2 (SAS Institute Inc, Cary, NC, USA).
Continuous variables are described as means, standard deviations (SDs), and the 95 % confidence interval (CI) of the mean. For comparisons from baseline to post-surgery, a two-sided t test was applied. The normality assumption was tested by a Shapiro–Wilk test to report a two-sided Wilcoxon signed-rank test if the normality assumption was violated. However, for all instances where the Shapiro–Wilk test indicated a significant deviation from normality, both the t test and the Wilcoxon signed-rank test were significant with P < 0.0001 for all comparisons reported. Statistical significance was defined as P ≤ 0.05. As secondary analysis, the differences in mean ODI score from baseline to 6, 12, and 24 months postoperatively was analysed with a repeated measurements analysis of variance (ANOVA) model. Changes in mean VAS score for back pain intensity were analysed over time in a similar manner. Both the absolute and relative frequencies of categorical variables were calculated.
Mean SF-36 PCS and MCS scores were calculated only for patients who completed ≥50 % of the items. For patients who completed ≥50 % of the items but who had missing values, the average score across the completed items was calculated to estimate the missing item values. Other outcomes with missing values were calculated without inclusion of a value for the patient in question.
Results
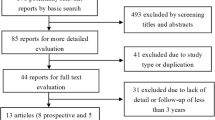
One hundred thirty-four patients with back pain were implanted with Maverick discs and followed for 2 years with 104 (78 %) evaluable patients remaining at 24 months (Fig. 1). The vast majority of patients complied with in- and exclusion criteria for Maverick disc replacement (Table 2). Because of the observational study design, intended to reflect routine practice, patients with extended indications were included in the analysis. The number of implants per patient, length of surgery, and blood loss observed during the study are described in Table 3. The lumbar spine was approached anteriorly through retroperitoneal exposure for most patients [n = 131; 98.5 %; transperitoneal: n = 2; 1.5 % (approach data are missing for one patient)].
Patient enrolment and follow-up. This study enrolled 139 patients. The number of patients with evaluable data from Maverick disc implantation (n = 134) through follow-up at 24 months (n = 104) is described. The bold boxes on the left describe the patients who had no evaluable data for the subsequent evaluations, whereas the boxes on the right describe those patients who missed visits, but completed subsequent assessments
Disability
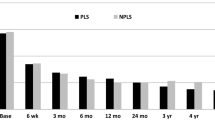
Mean (SD) ODI score was 50.1 (16.2) at baseline and 24.2 (18.5) at 6 months. Mean ODI score had reduced significantly by 25.4 points (patient with paired values: n = 122; 95 % CI −29.0 to −21.9; P < 0.0001; Fig. 2) and it continued to decrease through 24 months. According to FDA success criterion (≥15-point improvement in ODI) [9], 74.6 and 75.2 % of patients had successful outcomes at 6 and 24 months, respectively.
Reduction in disability. Disability was measured pre-operatively and at 6, 12, and 24 months postoperatively using the Oswestry Disability Index (ODI version 2.1). Compared with pre-operative mean ODI scores, all follow-up mean ODI scores were statistically significant (error bars depict the 95 % confidence intervals)
Pain and pain medication
Mean low back pain intensity scores (VAS) decreased significantly from baseline to 6 months (−4.0; 95 % CI −4.5 to −3.4; P < 0.0001), and further reductions were noted through 24 months (Fig. 3). Reductions in the intensity and frequency of leg pain over the same time period were also noted (Table 4).
Low back pain relief. Low back pain (LBP) intensity and frequency were assessed pre-operatively and at 6, 12, and 24 months postoperatively using the 10-cm visual analogue scale [VAS; 0 cm (no pain) to 10 cm (worst possible pain)]. Compared with pre-operative mean VAS scores, all follow-up mean VAS scores were statistically significant (error bars depict the 95 % confidence intervals)
The number of patients requiring non-drug pain interventions or pain medicine post-surgery was reduced (Table 4), as was the usage of all types of medications: nonopioids or nonsteroidal anti-inflammatory drugs, mild and strong opioids, neuropathic pain medications, and adjuvants.
Range of motion
Postoperatively, most patients with available radiographs had ≥3° of motion (extension–flexion) at the implant level: 84 % (53/63), 87 % (52/60), and 85 % (52/61) at 6, 12, and 24 months, respectively. By 6, 12, and 24 months, mean (SD) range of motion increased to 8.4˚ (4.4; 95 % CI 7.3–9.5), 8.4° (4.9; 95 % CI 7.1–9.7), and 9.4° (5.6; 95 % CI 8.0–10.9), respectively, from the mean pre-operative range of motion of 6.2° (4.8; 95 % CI 5.0–7.5).
Work status
At baseline, 58 patients (43 %) were not working, 55 of them (95 %) ascribing this to back problems. Six months post-surgery, this number increased to 75 (57 %) but only 49 patients (65 %) ascribed this to back problems. By 12 and 24 months, these numbers had reduced below baseline to 39 (37 %) and 36 (36 %), respectively, with 20 patients citing back problems. Figure 4 shows return to work information of professional workers who had to stop working due to back problems pre-surgery.
Return to work. Patients’ work status was assessed postoperatively through 24 months. Only professional workers who had to stop working before surgery are depicted below (not those who were retired, unemployed, a student, or a homemaker). The number of professional workers who returned to work post-surgery was divided by the total number of professional workers for which data on resuming work was available
Quality of life
Mean SF-36 PCS and MCS scores significantly increased postoperatively, with an 11.6-point and 8.8-point increase from baseline at 6 months, then to 14.4 and 9.8 at 24 months (P < 0.0001 for all; Fig. 5). 76 % of patients had a successful (≥4.9-point increase in PCS) outcome at 24 months.
Improvement in quality of life. Patient quality of life was measured pre-operatively and at 6, 12, and 24 months postoperatively using the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Short-Form Health Survey (SF-36) v2. Compared with pre-operative mean scores, all follow-up mean PCS and MCS scores were statistically significant (error bars depict the 95 % confidence intervals)
Patient satisfaction
Patients were asked (1) whether they would have the treatment again, and (2) whether they had completely recovered pre-operative health: they reported an overall positive perceived effect (Table 4).
Results of other tertiary outcomes are shown in Online Resource 1.
Adverse events
Fifty seven patients (42 %) experienced a complication or AE. AEs occurring within 6 months post-surgery are described in Table 5. Between 6 and 24 months, the following additional AEs occurred (in parentheses the number of events not resolved at study end): 13 late radiculopathies (10), 6 occurrences of late nonspecific low back pain (3), and 18 other events (10). One foreign body (allergic) reaction was observed during the 2-year follow-up period. Resolution of AEs included re-operation for abdominal wall weakness or haematoma for three patients and removal of the implant for the patient with the foreign body reaction for persisting pain. Two other surgeries—one on the foot, one on the hip—were not linked to the spine pathology.
Discussion
This prospective observational study examined the effectiveness of the Maverick TDR prosthesis under real-life surgical conditions in patients receiving routine standard care for DDD at their respective centres. It is the first international study reporting outcomes of TDR in a wide patient population treated under different national standards of care. To date, limited information derived from a national registry (Switzerland) is available on outcomes following TDR in patients treated outside a controlled clinical study setting [10, 12]. Two publications of TDR based on a national registry have reported reductions in low back pain at a mean follow-up time of 8 months [10] and 1 year [12]. Changes in disability, however, were not measured in these observational study settings.
Despite the uncontrolled nature of current study, the findings are similar to those of previous prospective randomised studies [2, 9, 14]. This registry demonstrated statistically significant improvements in its primary and secondary endpoints—disability (ODI) and back pain (VAS) scores 6 months postoperatively—which were maintained through 12- and 24-month follow-ups. Change in ODI met the criteria for a clinically important difference as defined by the FDA with 75.2 % of patients having ≥15-point improvement at 24 months [8]. In the randomised controlled trial (RCT) comparing Maverick disc implants versus interbody fusion, this was 82.2 % of the patients [9]. Mean low back pain scores observed in both studies underwent significant reductions from pre-operation to 6 and 24 months (registry: P < 0.0001; RCT: P < 0.001), although a direct comparison of the results is not possible because the RCT employed a 0–100 VAS scale and this study used a 0–10 scale [9]. Like the prospective randomised studies, reductions in pain and disability were attained in the first year of this study, and lower values maintained in the second year [2, 9, 14]. Changes from pre-operation to 6 and 24 months in mean ODI and VAS scores observed in this study are also comparable to other prospective studies of Maverick TDR, including the 4-year prospective study [4, 9]. Taken together, these findings suggest that Maverick TDR performed in current, real-life surgical settings can provide similar outcomes to those seen in trials.
The registry population had 64 % of patients that returned to work 2 years postoperatively. In the Maverick RCT, the multi-device RCT and the PRODisc prospective nonrandomised study, the percentages should be 68 %, 74 % and 76 %, respectively [3, 9, 14]. Only 20 % of this registry population ascribed their lack of work to back problems. The work status at 2 years in this registry was equivalent to that observed in the prospective, randomised investigational device exemption (IDE) CHARITE trial: 63 % [2]. Comparisons of work status across studies conducted at different times and in different locations, however, must be tempered by the influence of the current employment rate and local practice recommendation for post-surgical work hiatus, and type of study.
Like the Maverick RCT and the Maverick 4-year prospective study, this registry noted a significant increase in the patient’s quality of life from pre-surgery to 6 months and 2 years post-surgery [4, 9]. A total of 78 % of patients in the Maverick RCT reported that they had either completely recovered or were much improved after 2 years [9] compared with 81 % of reported improvements in this registry. Furthermore, 82 % of patients in this registry compared to 86 % of patients in the PRODisc study [3] and 73 % in the randomised IDE CHARITE trial reported that they would have the treatment again [2].
The incidence of AEs (42 %)—prompting reporting—during the 2-year follow-up in this observational study is lower than the rate observed in the Maverick RCT (>80 %) [9]. Overall complication rates reported in the published literature range from 1 to 40 % [3]. One case of allergic reaction was noted (first symptoms at 1 year) and the prosthesis was explanted. The Maverick RCT also reported one case of metallurgic allergy at 7 months [9]. This rare problem has been previously noted and is due to the creation of ionic species at the metallic articulating surfaces precipitating a cell-mediated hypersensitivity reaction (type IV) [15, 16].
Limitations of this study are related to the nature of multicentre observational non-comparative studies. These include variations in surgical centre standards of care, assessment, follow-up, and lack of a control group. At the same time, this “real-life” setting offers insight into the effectiveness of TDR for treating DDD with high external validity.
In conclusion, this international prospective observational study shows that TDR performed by experienced surgeons, leads to a statistically significant improvement in disability (ODI) and pain (VAS) scores at 6 months, which were maintained for 2 years, providing the first evidence of the effectiveness of TDR in real-world clinical settings across countries and patient populations, with an acceptable rate of perioperative complications. The similarity in outcomes of this registry compared to those from RCTs support the use of registry data for assessing interventions.
References
Le Huec JC, Mathews H, Basso Y et al (2005) Clinical results of Maverick lumbar total disc replacement: 2-year prospective follow-up. Orthop Clin North Am 36(3):315–322
Blumenthal S, McAfee PC, Guyer RD et al (2005) A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976) 30(14):1565–1575
Siepe CJ, Mayer HM, Wiechert K, Korge A (2006) Clinical results of total lumbar disc replacement with ProDisc II: 3-year results for different indications. Spine (Phila Pa 1976) 31(17):1923–1932
Van de Kelft E, Verguts L (2012) Clinical outcome of monosegmental total disc replacement for lumbar disc disease with ball-and-socket prosthesis (Maverick): prospective study with 4-year follow-up. World Neurosurg 78(3–4):355–363
Geisler FH, Blumenthal SL, Guyer RD et al (2004) Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charite intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1(2):143–154
Huang RC, Tropiano P, Marnay T, Girardi FP, Lim MR, Cammisa FP Jr (2006) Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J 6(3):242–247
Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG (2004) Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 86-A(7):1497–1503
Mathews HH, Le Huec JC, Friesem T, Zdeblick T, Eisermann L (2004) Design rationale and biomechanics of Maverick total disc arthroplasty with early clinical results. Spine J 4(6 Suppl):268S–275S
Gornet MF, Burkus JK, Dryer RF, Peloza JH (2011) Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine (Phila Pa 1976) 36(25):E1600–E1611
Zweig T, Aghayev E, Melloh M, Dietrich D, Roder C (2012) Influence of preoperative leg pain and radiculopathy on outcomes in mono-segmental lumbar total disc replacement: results from a nationwide registry. Eur Spine J 21(Suppl 6):S729–S736
Chin KR (2007) Epidemiology of indications and contraindications to total disc replacement in an academic practice. Spine J 7(4):392–398
Aghayev E, Henning J, Munting E, Diel P, Moulin P, Roder C (2012) Comparative effectiveness research across two spine registries. Eur Spine J 21(8):1640–1647
Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY (2008) Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 8(6):968–974
Berg S, Tullberg T, Branth B, Olerud C, Tropp H (2009) Total disc replacement compared to lumbar fusion: a randomised controlled trial with 2-year follow-up. Eur Spine J 18(10):1512–1519
Zairi F, Remacle JM, Allaoui M, Assaker R (2013) Delayed hypersensitivity reaction caused by metal-on-metal total disc replacement. J Neurosurg Spine 19(3):389–391
Gornet MF, Burkus JK, Harper ML, Chan FW, Skipor AK, Jacobs JJ (2013) Prospective study on serum metal levels in patients with metal-on-metal lumbar disc arthroplasty. Eur Spine J 22(4):741–746
Acknowledgments
The authors wish to thank the Medtronic clinical study manager Dr. Christian Pittet for conducting the MAVERICK study; Dr. Cristina Faria (clinical affairs manager), Drs. Kirstin Demesmaeker and Alexander Cristea (medical affairs managers), Dr. Mayra Mori (scientific communications specialist) and Monica Bromley for technical review of the manuscript. The authors also thank Bert Parmet and Jacques P. G. Janssen from CromSource for performing the statistical analysis and Dr. Rebecca Bachmann Quintiles for medical writing assistance. CromSource and Quintiles were contracted as independent providers and funded by Medtronic.
Conflict of interest
Medtronic grant funds were received to support the trial. Richard Assaker, Jean-Charles Le Huec, and Jörg Franke: Consultancy agreement with Medtronic. Other financial activities outside submitted work: Dominique Vardon and Jorg Franke: Medtronic honoraria for lectures. None of the authors received payment for writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Assaker and K. Ritter-Lang were co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assaker, R., Ritter-Lang, K., Vardon, D. et al. Maverick total disc replacement in a real-world patient population: a prospective, multicentre, observational study. Eur Spine J 24, 2047–2055 (2015). https://doi.org/10.1007/s00586-015-3918-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3918-x