Abstract
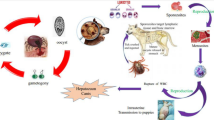
Hepatozoon species (phylum Apicomplexa: Adeleorina, Hepatozooidae) are the cause of canine hepatozoonosis, a vector-borne disease (VBD) spread by ticks (Ixodidae). The aim of the present study was to determine the prevalence of Hepatozoon canis (H. canis) infections in different pet dogs admitted to different clinics in Egypt as well as determine the effect of H. canis on dogs through analysis of the immunogenic genes and the oxidative stress markers. One hundred dogs with different clinical signs, fever, fatigue, tick infestation around the ears and neck region, were examined. Thin blood films were performed and stained with Giemsa. Thirty examined dogs (30%) were positive for H. canis by direct observation of the gamonts in circulating leucocytes on blood smears stained with Giemsa. Two hundred ticks were identified morphologically and all ticks were classified as R. sanguineus. Hematological and biochemical results of sampled dogs were recorded. The AST and ALT levels were higher than control-negative healthy dogs. MDA levels in H. canis infected dogs were higher than that of control negative dogs. The transcript levels of the different targeted genes (IL-1β; IL6; TNF-α and IFN-γ) were upregulated in infected dogs with H. canis significantly than control healthy dogs. Canine hepatozoonosis induced tissue reaction evaluated by different immunological genes and oxidative stress.





Similar content being viewed by others
Data availability
All data presented in the manuscript; no additional data were used for the research described in this article.
References
AbdElKader NA, Sheta E, AbuBakr HO et al (2020) Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: gasterophilidae) larvae in donkeys (Equus asinus). Int J Trop Insect Sci. https://doi.org/10.1007/s42690-020-00171-2
Aktas M, Özübek S, Altay K, Balkaya I, Utuk AE, Kırbas A, Şimsek S, Dumanlı N (2015) A molecular and parasitological survey of Hepatozoon canis in domestic dogs in Turkey. Vet Parasitol 209(3–4):264–267. https://doi.org/10.1016/j.vetpar.2015.02.015
Attia MM, El-Gameel SM, Ismael E (2020) Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J Parasit Dis 44(2):332–337. https://doi.org/10.1007/s12639-020-01220-w
Attia MM, Abdelsalam M, Korany RMS, Mahdy OA (2021a) Characterization of digenetic trematodes infecting African catfish (Clarias gariepinus) based on integrated morphological, molecular, histopathological, and immunological examination. Parasitol Res 120(9):3149–3162. https://doi.org/10.1007/s00436-021-07257-x
Attia MM, Elgendy MY, Prince A, El‐Adawy MM, Abdelsalam M (2021b) Morphomolecular identification of two trichodinid coinfections (Ciliophora: Trichodinidae) and their immunological impacts on farmed Nile Tilapia. Aquaculture Res
Attia MM, Salem HM (2022) Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int J Trop Insect Sci 42(1):733–740
Attipa C, Maguire D, Solano-Gallego L, Szladovits B, Barker EN, Farr A, Baneth G, Tasker S (2018) Hepatozoon canis in three imported dogs: a new tick-borne disease reaching the United Kingdom. Veterinary Record. https://doi.org/10.1136/vr.105087
Baneth G, Mathew JS, Shkap V, Macintire DK, Barta JR, Ewing SA (2003) Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol 19:27–31
Barati A, Razmi GR (2018) A parasitologic and molecular survey of Hepatozoon canis infection in stray dogs in Northeastern Iran. J Parasitol 104(4):413–417. https://doi.org/10.1645/17-105. Epub 15 May 2018
Dąbrowski R, Wdowiak A, Szczubiał M, Krakowski L, Brodzki P, Bochniarz M, Tvarijonaviciute A (2020) Changes in interferon-gamma and neopterin in female dogs undergoing ovariohysterectomy as elective spay or as treatment of pyometra. Can J Vet Res = Revue canadienne de recherche veterinaire 84(3):230–234
Dinarello CA (1996) Biologic basis for interleukin-1 in disease”. Blood 87(6):2095–2147
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117(14):3720–3732. https://doi.org/10.1182/blood-2010-07-273417
Díaz-Sánchez AA, Hofmann-Lehmann R, Meli ML, Roblejo-Arias L, Fonseca-Rodríguez O, Castillo AP, Cañizares EV, Rivero EL, Chilton NB, Corona-González B (2021) Molecular detection and characterization of Hepatozoon canis in stray dogs from Cuba. Parasitol Int 80:102200. https://doi.org/10.1016/j.parint.2020.102200
El-Dakhly KM, Goto M, Noishiki K, El-Nahass S, Hirata A, Saka H, Takashima Y, El- Morsey A, Yanai T (2013) Prevalence and diversity of Hepatozoon canis in naturally infected dogs in Japanese islands and peninsulas. Parasitol Res 112:3267–3274
Giannelli A, Lia RP, Annoscia G, Buonavoglia C, Lorusso E, DantasTorres F, Baneth G, Otranto D (2017) Rhipicephalus turanicus, a new vector of Hepatozoon canis. Parasitology 144:730–737
Giannelli A, Ramos RA, Dantas-Torres F, Mencke N, Baneth G, Otranto D (2013) Experimental evidence against transmission of Hepatozoon canis by Ixodes ricinus. Ticks Tick Borne Dis 4:391–394. https://doi.org/10.1016/j.ttbdis.2013.03.001
Hegab AA, Omar HM, Abuowarda M, Ghattas SG, Mahmoud NE, Fahmy MM (2022) Screening and phylogenetic characterization of tick-borne pathogens in a population of dogs and associated ticks in Egypt. Parasit Vectors 15:222. https://doi.org/10.1186/s13071-022-05348-x
Jiménez AG (2023) Inflammaging in domestic dogs: basal level concentrations of IL-6, IL-1β, and TNF-α in serum of healthy dogs of different body sizes and ages. Biogerontology 24(4):593–602. https://doi.org/10.1007/s10522-023-10037-y
Kaura N, Singha H, Sharmaa P, Singh NK, Kashyapc N, Singha NK (2020) Development and application of multiplex PCR assay for the simultaneous detection of H. canisvogeli, Ehrlichiacanis and Hepatozoon canis in dogs. Acta Trop 212:105713. https://doi.org/10.1016/j.actatropica.2020.105713
Kiral F, Karagenc T, Pasa S, Yenisey C, Seyrek K (2005) Dogs with Hepatozoon canis respond to the oxidative stress by increased production of glutathione and nitric oxide. Vet Parasitol 131(1–2):15–21. https://doi.org/10.1016/j.vetpar.2005.04.017
Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML (1995) Induction of interleukin-6 synthesis in the myocardium: potential role in postreperfusion inflammatory injury. Circulation 92(7):1866–1875
Kwon SJ, Kim YH, Oh HH, Choi US (2017) First case of canine infection with Hepatozoon canis (Apicomplexa: Haemogregarinidae) in the Republic of Korea. Korean J Parasitol 55(5):561–564. https://doi.org/10.3347/kjp.2017.55.5.561
Lakshmanan B, Jose J, George A, Usha NP, Devada K (2018) Molecular detection of Hepatozoon canis in dogs from Kerala. J Parasit Dis 42(2):287–290. https://doi.org/10.1007/s12639-018-0998-7
Léveillé AN, Baneth G, Barta JR (2019) Next generation sequencing from Hepatozoon canis (Apicomplexa: Coccidia: Adeleorina): complete apicoplast genome and multiple mitochondrion-associated sequences. Int J Parasitol 49:375–387
Li Y, Wang C, Allen KE, Little SE, Ahluwalia SK, Gao D, Macintire DK, Blagburn BL, Kaltenboeck B (2008) Diagnosis of canine Hepatozoon spp. infection by quantitative PCR. Vet Parasitol 1157(1–2):50–8. https://doi.org/10.1016/j.vetpar.2008.06.027
Maissen-Villiger CA, Schweighauser A, van Dorland HA, Morel C, Bruckmaier RM, Zurbriggen A, Francey T (2016) Expression profile of cytokines and enzymes mRNA in blood leukocytes of dogs with leptospirosis and its associated pulmonary hemorrhage syndrome. PLoS One 11(1):e0148029
Mankowska M, Stachowiak M, Graczyk A, Ciazynska P, Gogulski M, Nizanski W, Switonski M (2016) Sequence analysis of three canine adipokine genes revealed an association between TNF polymorphisms and obesity in Labrador dogs. Anim Genet 47:245–249. Published online December 22, 2015
Murata T, Inoue M, Tateyama S, Taura Y, Nakama S (1993) Vertical transmission of Hepatozoon canis in dogs. J Vet Med Sci 55:867–868
Orkun Ö, Koç N, Sürsal N, Çakmak A, Nalbantoğlu S, Karaer Z (2018) Molecular characterization of tick-borne blood protozoa in stray dogs from Central Anatolia Region of Turkey with a high-rate Hepatozoon infection. KafkasUniv Vet FakDerg 24(2):227–232. https://doi.org/10.9775/kvfd.2017.18678
Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, Stanneck D, de Caprariis D, Capelli G, Baneth G (2011) Diagnosis of Hepatozoon canis in young dogs by cytology and PCR. Parasit Vectors 4:55. https://doi.org/10.1186/1756-3305-4-55
Prachar C, Kaup F, Neumann S (2013) Interleukin-1 beta (IL-1β) in the peripheral blood of dogs as a possible marker for the detection of early stages of inflammation. Open Journal of Veterinary Medicine 3(7):302–308. https://doi.org/10.4236/ojvm.2013.37049
Salakij C, Salakij J, Rochanapat N, Suthunmapinunta P, Nunklang G (1999) Hematological characteristics of blood parasite infected dogs. Kasetsart J Nat Sci 33(4):589–600
Salem HM, Khattab MS, Yehia N, Abd El-Hack ME, El-Saadony MT, Alhimaidi AR, Swelum AA, Attia MM (2021) Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult Sci 101
Sarma K, MondalD B, Saravanan M, Kumar M, Mahendran K (2012) Haemato-biochemical changes in Hepatozoon canis infected dog before and after therapeutic management. J Veterinary Parasitol 26(1):35–38
Soulsby EJL (1986) Helminths, arthropods and protozoa of domesticated animals,7th edn. Bailliere Tindall, London. 167–174
Thongsahuan S, Chethanond U, Wasiksiri S, Saechan V, Thongtako W, Musikacharoen T (2020) Hematological profile of blood parasitic infected dogs in Southern Thailand. Vet World 13(11):2388–2394. https://doi.org/10.14202/vetworld.2020.2388-2394
Zaki AA, Attia MM, Ismael E, Mahdy OA (2021) Prevalence, genetic, and biochemical evaluation of immune response of police dogs infected with Babesia vogeli. Vet World 14(4):903–912
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the sampling from animals was conducted under local Ethical Committee laws and regulations as regard to the care and use of laboratory animals Vet CU 01122022624.
Informed consent
For this type of study, informed consent is not required.
Consent for publication
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalifa, M.M., Attia, M.M. Pathogenic effects of Hepatozoon canis (Apicomplexa: Hepatozoidae) on pet dogs (Canis familiaris) with amplification of immunogenetic biomarkers. Comp Clin Pathol 33, 223–230 (2024). https://doi.org/10.1007/s00580-023-03542-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03542-6