Abstract
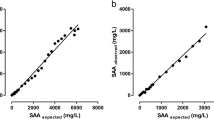
Serum amyloid A (SAA) is one of the most important acute phase proteins in cats and increases rapidly and significantly after an inflammatory stimulus. The objective of this study was to compare measurements of SAA concentration in feline blood specimens using the point-of-care Eurolyser Solo Analyzer (SOLO) with results of a validated immunoturbidimetric test. This prospective study was conducted between March 2014 and June 2015 at a university teaching hospital. Blood specimens were collected from a total of 61 cats including 45 client-owned cats with inflammatory diseases presenting to the emergency and critical care service and 16 client-owned healthy cats. Before SAA measurement, the blood was centrifuged and stored at − 80 °C (− 112 °F). The Eiken assay immunoturbidimetric method (EKITM) which uses latex sensitized anti-human SAA antibodies served as reference SAA test. Serum amyloid A measurements with the EKITM were performed in a reference laboratory. Serum amyloid A measurements with the SOLO were performed in an emergency laboratory. Thirty-six and 18 results were out of the analytical range with the EKITM and the SOLO, respectively. The correlation between the two methods was high (r = 0.87) and there was an intense mostly proportional bias, with results being approximately 2 times lower with the SOLO than the reference test. This study showed that the SOLO reliably and rapidly measures SAA in feline blood specimens; however, a “de novo” reference interval should be determined for proper interpretation of the results.


Similar content being viewed by others
Notes
Solo, Eurolyser Diagnostica GmbH, Salzburg, Austria
Eiken assay LZ test SAA, Eiken Chemical Co, Tokyo, Japan, lot n°46,007
Venosafe® 3 mL, Terumo, Europe N.V, Leuven, Belgium
SIGMA 3 K10® Laborzentrifugen, Bioblock Scientific
VT 1700 SAA Control, Solo, Eurolyser Diagnostica GmbH, Austria - mean and range of acceptability: 101 mg / L and 66–136 mg / L respectively
Abbott Architect c4000, Abbott Park Road, Libertyville Township, IL, USA
Analyse-It, Leeds, UK
References
Brady CA, Otto CM, Van Winkle TJ, King LG (2000) Severe sepsis in cats: 29 cases (1986-1998). J Am Vet Med Assoc 217:531–535
Carey RN, Anderson FP, George H et al. (2005) User verification of performance for precision and trueness; approved guideline, 2nd edn. NCCLS Document EP15-A2, Clinical and Laboratory Standards Institute, Wayne, p 1–49
Ceron JJ, Eckersall PD, Martynez-Subiela S (2005) Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 34:85–99
Ceron JJ (2008) A seven-point plan for acute phase protein interpretation in companion animals. Vet J 177:6–7
Christensen M, Jacobsen S, Ichiyanagi T, Kjelgaard-Hansen M (2012) Evaluation of an automated assay based on monoclonal anti-human serum amyloid A (SAA) antibodies for measurement of canine, feline, and equine SAA. Vet J 194:332–337
Friedrichs KR, Harr KE, Freeman KP et al (2012) ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453
Geffre A, Friedrichs K, Harr K et al (2009) Reference values: a review. Vet Clin Pathol 38:288–298
Hansen AE, Schaap MK, Kjelgaard-Hansen M (2006) Evaluation of a commercially available human serum amyloid A (SAA) turbidimetric immunoassay for determination of feline SAA concentration. Vet Res Commun 30:863–872
Harr KE, Flatland B, Nabity M et al (2013) ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 42:424–436
Horowitz GL, Altaie S, Boyd JC et al (2008) Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, 3rd edn. CLSI Document C28-A3, Clinical and Laboratory Standards Institute, Wayne
Jensen AL, Kjelgaard-Hansen M (2006) Method comparison in the clinical laboratory. Vet Clin Pathol 35:276–286
Kajikawa T, Furuta A, Onishi T, Sugii S (1996) Enzyme-linked immunosorbent assay for detection of feline serum amyloid A protein by use of immunological cross-reactivity of polyclonal anti-canine serum amyloid A protein antibody. J Vet Med Sci 58:1141–1143
Kajikawa T, Furuta A, Onishi T et al (1999) Changes in concentrations of serum amyloid A protein, alpha 1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet Immunol Immunopathol 68:91–98
Kann RKC, Seddon JM, Henning J et al (2012) Acute phase proteins in healthy and sick cats. Res Vet Sci 93:649–654
Ruthrauff CM, Smith J, Glerum L (2009) Primary bacterial septic peritonitis in cats: 13 cases. J Am Vet Med Assoc 45:268–276
Sasaki K, Ma Z, Khatlani TS et al (2003) Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J Vet Med Sci 65:545–548
Segev G, Klement E, Aroch I (2006) Toxic neutrophils in cats: clinical and clinicopathologic features, and disease prevalence and outcome—a retrospective case control study. J Vet Intern Med 20:20–31
Sergeeff JS, Armstrong PJ, Bunch SE (2004) Hepatic abscesses in cats: 14 cases (1985-2002). J Vet Intern Med 18:295–300
Solter PF, Hoffmann WE, Hungerford LL et al (1991) Haptoglobin and ceruloplasmin as determinants of inflammation in dogs. Am J Vet Res 52:1738–1742
Tamamoto T, Ohno K, Takahashi M et al (2013) Serum amyloid A as a prognostic marker in cats with various diseases. J Vet Diagn Investig 25:428–432
Acknowledgements
The authors are grateful to Dr. Anna Hillström, DVM, PhD, DECVCP, for her help in original data collection and in the preparation of the present manuscript.
Funding
Solo, Eurolyser Diagnostica GmbH, Salzburg, Austria, and reagents were provided by Scil Vet, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Gaillard, E., Aumann, M., Leynaud, V. et al. Comparison of feline serum amyloid A (SAA) measurements assessed by a point-of-care test analyzer to a validated immunoturbidimetric method. Comp Clin Pathol 27, 321–325 (2018). https://doi.org/10.1007/s00580-017-2593-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2593-1