Abstract
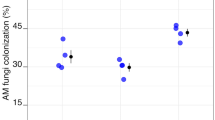
Effects of the arbuscular mycorrhizal (AM) fungus Rhizophagus irregularis on plant growth, carbon (C) and nitrogen (N) accumulation, and partitioning was investigated in Triticum aestivum L. plants grown under elevated CO2 in a pot experiment. Wheat plants inoculated or not inoculated with the AM fungus were grown in two glasshouse cells with different CO2 concentrations (400 and 700 ppm) for 10 weeks. A 15N isotope labeling technique was used to trace plant N uptake. Results showed that elevated CO2 increased AM fungal colonization. Under CO2 elevation, AM plants had higher C concentration and higher plant biomass than the non-AM plants. CO2 elevation did not affect C and N partitioning in plant organs, while AM symbiosis increased C and N allocation into the roots. In addition, plant C and N accumulation, 15N recovery rate, and N use efficiency (NUE) were significantly higher in AM plants than in non-AM controls under CO2 enrichment. It is concluded that AM symbiosis favors C and N partitioning in roots, increases C accumulation and N uptake, and leads to greater NUE in wheat plants grown at elevated CO2.




Similar content being viewed by others
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371
Aljazairi S, Arias C, Nogues S (2015) Carbon and nitrogen allocation and partitioning in traditional and modern wheat genotypes under pre-industrial and future CO2 conditions. Plant Biol 17:647–659
Baslam M, Erice G, Goicoechea N (2012) Impact of arbuscular mycorrhizal fungi (AMF) and atmospheric CO2 concentration on the biomass production and partitioning in the forage legume alfalfa. Symbiosis 58:171–181
Baslam M, Antolin MC, Gogorcena Y, Munoz F, Goicoechea N (2014) Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. Ann Appl Biol 164:190–199
Bloom AJ, Burger M, Kimball BA, Pinter PJ (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Chang 4:437–440
Bunce JA (2001) Direct and acclamatory responses of stomatal conductance to elevated carbon dioxide in four herbaceous crop species in the field. Glob Chang Biol 7:323–331
Cavagnaro TR, Sokolow SK, Jackson LE (2007) Mycorrhizal effects on growth and nutrition of tomato under elevated atmospheric carbon dioxide. Funct Plant Biol 34:730–736
Cavagnaro TR, Gleadow RM, Miller RE (2011) Plant nutrient acquisition and utilization in a high carbon dioxide world. Funct Plant Biol 38:87–96
Chen X, Tu C, Burton MG, Watson DM, Burkey KO, Hu S (2007) Plant nitrogen acquisition and interactions under elevated carbon dioxide: impact of endophytes and mycorrhizae. Glob Chang Biol 13:1238–1249
Cheng L, Bllker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084
Compant S, van der Heijden MGA, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol Ecol 73:197–214
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639
Drigo B, Kowalchuk GA (2013) Rhizosphere responses to elevated CO2. In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere. Volume 1 & 2. John Wiley & Sons, Inc, Hoboken
Drigo B, Pijl AS, Duyts H, Kielak AM, Gamper HA, Houtekamer MJ, Boschker HTS, Bodelier PLE, Whiteley AS, van Veen JA, Kowalchuk GA (2010) Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc Natl Acad Sci U S A 107:10938–10942
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bucking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 109:2666–2671
Gamper H, Hartwig UA, Leuchtmann A (2005) Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 yr of selection under elevated atmospheric CO2 partial pressure. New Phytol 167:531–542
Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I (2000) Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum L.) plants. J Exp Bot 51:1931–1938
Gianinazzi S, Gollotte A, Binet M, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Goicoechea N, Baslam M, Erice G, Irigoyen JJ (2014) Increased photosynthetic acclimation in alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated CO2. J Plant Physiol 171:1774–1781
Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bucking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
He Z, Xiong J, Kent AD, Deng Y, Xue K, Wang G, Wu L, Van Nostrand JD, Zhou J (2014) Distinct responses of soil microbial communities to elevated CO2 and O3 in a soybean agro-ecosystem. ISME J 8:714–726
IPCC (2013) Fifth assessment report on climate change 2013: the physical science basis. Final draft underlying scientific-technical assessment. Intergovernmental Panel on Climate Change, Working group l, Geneva
Johnson NC, Gehring CA (2007) Mycorrhizas: symbiotic mediators of rhizosphere and ecosystems processes. In: Cardon ZG, Whitback J (eds) The rhizosphere: an ecological perspective. Elsevier, New York, pp 73–100
Kormanik PP, Bryan WC, Schultz RC (1980) Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can J Microbiol 26:536–538
Liu ZL, Li YJ, Hou HY, Zhu XC, Rai V, He XY, Tian CJ (2013) Differences in the arbuscular mycorrhizal fungi-improved rice resistance to low temperature at two N levels: aspects of N and C metabolism on the plant side. Plant Physiol Biochem 71:87–95
Mitsutoshi K, Takayoshi K, Hiroyuki T, Yutaka M (2005) Elevated CO2 and limited nitrogen nutrition can restrict excitation energy dissipation in photosystem II of Japanese white birch (Betula platyphylla var. japonica) leaves. Physiol Plant 125:64–73
Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, Taylor M, Witt CA (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol 10:3–19
NOAA-ESRL (2015) National oceanic and atmospheric administration-earth system research laboratory. Atmospheric CO2 for Feb. 2015. http://co2now.org/Current-CO2/CO2-Now/
Pacholski A, Manderscheid R, Weigel HJ (2015) Effects of free air CO2 enrichment on root growth of barley, sugar beet and wheat growth in a rotation under different nitrogen supply. Eur J Agron 63:36–46
Rillig MC, Wright SF, Allen MF, Field CB (1999) Rise in carbon dioxide changes soil structure. Nature 400:628
Rillig MC, Treseder KK, Allen MF (2002) Global change and mycorrhizal fungi. In: van der Heijden MGA, Sanders I (eds) Mycorrhizal ecology. Springer Verlag, Berlin, pp 135–160
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Staddon PL (2005) Mycorrhizal fungi and environmental change: the need for a mycocentric approach. New Phytol 167:635–637
Staddon PL, Fitter AH, Robinson D (1999) Effects of mycorrhizal colonization and elevated atmospheric carbon dioxide on carbon fixation and below-ground carbon partitioning in Plantago lanceolata. J Exp Bot 50:853–860
Treseder KK, Egerton-Warburton LM, Allen MF, Cheng Y, Oechel WC (2003) Alteration of soil carbon pools and communities of mycorrhizal fungi in chaparral exposed to elevated carbon dioxide. Ecosystems 6:786–796
Xu Z, Shimizu H, Yagasaki Y, Ito S, Zheng Y, Zhou G (2013) Interactive effects of elevated CO2, drought, and warming on plants. J Plant Growth Regul 32:692–707
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zavalloni C, Vicca S, Buscher M, de la Providencia IE, de Boulois HD, Declerck S, Nijs I, Ceulemans R (2012) Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant Soil 359:121–136
Zhu XC, Song FB, Liu FL, Liu SQ, Tian CJ (2015) Carbon and nitrogen metabolism in arbuscular mycorrhizal maize plants under low-temperature stress. Crop Pasture Sci 66(1):62–70
Acknowledgments
This study was financially supported by the Key Research Program of the Chinese Academy of Sciences (KZZD-EW-TZ-16) and the China Scholarship Council. We are thankful for the assistance provided by Xiangnan Li and Fan Fan in the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Song, F., Liu, S. et al. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2 . Mycorrhiza 26, 133–140 (2016). https://doi.org/10.1007/s00572-015-0654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0654-3