Abstract
Background
This study was designed to investigate the pharmacokinetics and pharmacodynamics of dexmedetomidine in morbidly obese patients undergoing laparoscopic surgery.
Methods
Morbidly obese (body mass index ≥40 kg/m2) and normal weight patients scheduled for elective laparoscopic surgery were included (n = 8, each group). After baseline hemodynamic measurement, dexmedetomidine 1 μg/kg was administered over 10 min. General anesthesia was induced with propofol 1.5 mg/kg and fentanyl 4 μg/kg 20 min after completion of dexmedetomidine infusion; the lungs were mechanically ventilated after tracheal intubation. The pharmacokinetics of dexmedetomidine was analyzed by a noncompartment model. Hemodynamic data and peripheral oxygen saturation (SpO2) were measured up to 30 min after starting dexmedetomidine infusion. Sedation level was measured with the Observer’s Assessment of Alertness/Sedation (OAA/S) scale.
Results
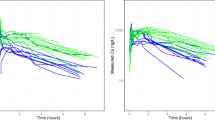
Peak plasma concentration, area under the curve to infinity, elimination half-life, and apparent volume of distribution were significantly larger in morbidly obese than in normal weight patients (3.75 ± 0.56 vs. 2.54 ± 0.32 µg/l, P < 0.001; 2174 ± 335 vs. 1594 ± 251 ng h/l, P < 0.001; 225 ± 55 vs. 158 ± 53 min, P = 0.02; 310 ± 63 vs. 164 ± 41 l, P < 0.001, respectively). Although clearance was also higher in obese patients than in normal body weight patients (58.6 ± 10.7 vs. 44.9 ± 9.0 l/h, P = 0.02), it was lower in obese patients than in normal body weight patients after normalization to total body weight (0.47 ± 0.07 vs. 0.64 ± 0.09 l/h/kg, P < 0.001). There were no differences in systolic or diastolic blood pressure or heart rate between the two groups within the 30 min. Sedation level was deeper and SpO2 was lower in morbidly obese than in normal weight patients. More patients in the morbidly obese patient group experienced deeper sedation after the start of the dexmedetomidine infusion (P < 0.05).
Conclusion
The pharmacokinetics and pharmacodynamics of dexmedetomidine are significantly different in morbidly obese patients compared with normal weight patients. Level of sedation was significantly deeper, and oxygen saturation was significantly lower, in morbidly obese than in normal weight patients, probably resulting from higher plasma concentration after infusion of 1.0 µg/kg.
Clinical trial number, registry URL
ClinicalTrials.gov (NCT01864187), https://register.clinicaltrials.gov/prs/app/action/LoginUser?ts=1&cx=-jg9qo4.




Similar content being viewed by others
References
Ingrande J, Lemmens HJ. Anesthetic pharmacology and the morbidly obese patient. Curr Anesthesiol Rep. 2013;3:10–7.
Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anesthesiol. 2012;62:118–33.
Dere K, Sucullu I, Budak ET, Yeyen S, Filiz AI, Ozkan S, Dagli G. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010;27:648–52.
Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics (Sao Paulo). 2014;69:777–86.
Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, Skrivanek G, Macaluso A, Shah M, Provost DA. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106:1741–8.
Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8.
Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41.
Wildman RP, Gu D, Muntner P, Wu X, Reynolds K, Duan X, Chen CS, Huang G, Bazzano LA, He J. Trends in overweight and obesity in Chinese adults: between 1991 and 1999–2000. Obesity (Silver Spring). 2008;16:1448–53.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87.
Akinnusi ME, Pineda LA, El SA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–8.
Lee S, Kim BH, Lim K, Stalker D, Wisemandle W, Shin SG, Jang IJ, Yu KS. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine in healthy Korean subjects. J Clin Pharm Ther. 2012;37:698–703.
Cortínez LI, Anderson BJ, Holford NHG, et al. Dexmedetomidine pharmacokinetics in the obese. Eur J Clin Pharmacol. 2015;71(12):1501–8.
Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, Young CC, Wright DR, Macleod DB, Somma J. Dexmedetomidine pharmacodynamics: part I. Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76.
Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–5.
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94.
Xiaoming D, Shanglong Y, Buwei Y, Yuguang H (2014) Modern anesthesiology, 4th edn. Beijing: People’s Health Publishing House (in Chinese).
Kohli U, Pandharipande P, Muszkat M, Sofowora GG, Friedman EA, Scheinin M, Wood AJ, Ely EW, Tyndale RF, Choi L, Stein CM, Kurnik D. CYP2A6 genetic variation and dexmedetomidine disposition. Eur J Clin Pharmacol. 2012;68(6):937–42.
Package insert # 3003391B. Precedex® product label. Spring Valley, NY: Par Pharm Laboratories; 2014.
Author information
Authors and Affiliations
Contributions
BX participated in study design, data collection, and provided the critical revision. DZ participated in literature search, data collection, data analysis, data interpretation, and wrote the manuscript. SS participated in data analysis, data interpretation, and wrote the manuscript. LR carried out the data collection and analysis. XZ conceived the study and participated in its design and coordination. MX participated in study design, data analysis, and data interpretation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Funding
Supported by the National Natural Science Foundation of China (Grant No. 61773130), the Special Project for Medical Science Development of PLA (Grant No. CGZ15C003), the Foundation for Sci & Tech Planning Project of Guangdong Province (Grant No. 2014A020215026).
Ethical approval
This manuscript describes human research.
IRB contact information: Guangzhou General Hospital of Guangzhou Military Command Medical Ethics Committee (Tel: +86-20-88653625, E-mail: gzjqzyy@163.com)
The study was registered before patient enrollment.
This report describes an observational clinical study.
The author states that the report includes every item in the STROBE checklist for case-control observational clinical studies.
This manuscript was not screened for plagiarism.
Informed consent
This study was conducted with written informed consent from the study subjects.
About this article
Cite this article
Xu, B., Zhou, D., Ren, L. et al. Pharmacokinetic and pharmacodynamics of intravenous dexmedetomidine in morbidly obese patients undergoing laparoscopic surgery. J Anesth 31, 813–820 (2017). https://doi.org/10.1007/s00540-017-2399-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2399-y