Abstract
Purpose
Postoperative nausea and vomiting (PONV) is a common complication after craniotomy. Vomiting may be a potentially hazardous complication in neurosurgical patients. We compared the efficacy of fosaprepitant and droperidol for the prevention of PONV, vomiting in particular, after craniotomy.
Methods
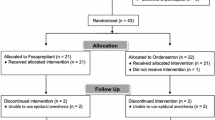
Patients scheduled to undergo elective craniotomy were enrolled in the study and randomly divided in a double-blind manner into two groups to receive either 150 mg of fosaprepitant (group F) or 1.25 mg of droperidol (group D). Dexamethasone (9.9 mg) was given to all patients, except those with diabetes mellitus. The incidence of PONV, frequency of vomiting, nausea score, and use of rescue antiemetic during the first 72 h after surgery were assessed at five time intervals (0–2, 2–6, 6–24, 24–48, and 48–72 h).
Results
Of the 200 randomized patients eligible for entry into the study, 186 were ultimately included for analysis. There were no significant differences in demographics or intraoperative variables between the two treatment groups. Over the entire 72-h post-craniotomy observation period the overall and cumulative incidence of vomiting was significantly lower in group F patients than in group D patients, while there were no between-group differences in the overall and cumulative incidence of PONV or in complete response (no PONV and no rescue antiemetic). The incidence and frequency of vomiting during each of the five observational periods were significantly lower in group F patients than group D patients, although there were no differences in the nausea score and antiemetic use between the groups.
Conclusion
Based on the results, fosaprepitant was more effective than droperidol in the prevention of vomiting after craniotomy over the entire 72-h study period. However, there was no difference in the incidence of nausea and antiemetic use.



Similar content being viewed by others
References
Fabling JM, Gan TJ, EL-Moalem HE, Warner DS, Borel CO. A randomized, double-blinded comparison of ondansetron, droperidol, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. Anesth Analg. 2000;91:358–61.
Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43.
Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011;112:813–8.
Tsutsumi YM, Kakuta N, Soga T, Kume K, Hamaguchi E, Tsutsumi R, Tanaka K. The effects of intravenous fosaprepitant and ondansetron for the prevention of postoperative nausea and vomiting in neurosurgery patients: a prospective, randomized, double-blinded study. Biomed Res Int. 2014;2014:307025.
Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, Habib AS, Knighton J, Carides AD, Zhang H, Horgan KJ, Evans JK, Lawson FC, Aprepitant-PONV Study Group. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9.
Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, Pueyo J, Chelly JE, Carides AD, Reiss T, Evans JK, Lawson FC, Aprepitant-PONV Protocol 091 International Study Group. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99:202–11.
Soga T, Kume K, Kakuta N, Hamaguchi E, Tsutsumi R, Kawanishi R, Fukuta K, Tanaka K, Tsutsumi YM. Fosaprepitant versus ondansetron for the prevention of postoperative nausea and vomiting in patients who undergo gynecologic abdominal surgery with patient-controlled epidural analgesia: a prospective, randomized, double-blind study. J Anesth. 2015;29:696–701.
Lee SJ, Lee SM, Kim SI, Ok SY, Kim SH, Park SY, Kim MG. The effect of aprepitant for the prevention of postoperative nausea and vomiting in patients undergoing gynecologic surgery with intravenous patient controlled analgesia using fentanyl: aprepitant plus ramosetron vs ramosetron alone. Korean J Anesthesiol. 2012;63:221–6.
Kakuta N, Tsutsumi YM, Horikawa YT, Kawano H, Tanaka K, Oshita S. Neurokinin-1 receptor antagonism, aprepitant, effectively diminishes post-operative nausea and vomiting while increasing analgesic tolerance in laparoscopic gynecological procedures. J Med Invest. 2011;58:246–51.
Kakuta N, Kume K, Hamaguchi E, Tsutsumi R, Mita N, Tanaka K, Tsutsumi YM. The effects of intravenous fosaprepitant and ondansetron in the prevention of postoperative nausea and vomiting in patients who underwent lower limb surgery: a prospective, randomized, double-blind study. J Anesth. 2015;29:836–41.
Vallejo MC, Phelps AL, Ibinson JW, Barnes LR, Milord PJ, Romeo RC, Williams BA, Sah N. Aprepitant plus ondansetron compared with ondansetron alone in reducing postoperative nausea and vomiting in ambulatory patients undergoing plastic surgery. Plast Reconstr Surg. 2012;129:519–26.
Trimas SJ, Trimas MD. Use of aprepitant and factors associated with incidence of postoperative nausea and vomiting in patients undergoing facial plastic surgery. JAMA Facial Plast Surg. 2015;17:251–5.
Kawano H, Matsumoto T, Hamaguchi E, Manabe S, Nakagawa M, Yamada A, Fujimoto M, Tada F. Antiemetic efficacy of combined aprepitant and dexamethasone in patients at high-risk of postoperative nausea and vomiting from epidural fentanyl analgesia. Minerva Anestesiol. 2015;81:362–8.
Lim CS, Ko YK, Kim YH, Park SI, Kim JK, Kim MJ, Kim HJ. Efficacy of the oral neurokinin-1 receptor antagonist aprepitant administered with ondansetron for the prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2013;64:212–7.
Sinha AC, Singh PM, Williams NW, Ochroch EA, Goudra BG. Aprepitant’s prophylactic efficacy in decreasing postoperative nausea and vomiting in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2014;24:225–31.
Tran LH, Cudny M, Ngo D, Patel S, Lam MY. Fosaprepitant for the treatment of refractory postoperative nausea and vomiting. Hosp Pharm. 2015;50:221–3.
Singh PM, Borle A, Rewari V, Makkar JK, Trikha A, Sinha AC, Goudra B. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J. 2016;92:87–98.
Milnes V, Gonzalez A, Amos V. Aprepitant: a new modality for the prevention of postoperative nausea and vomiting: an evidence-based review. J Perianesth Nurs. 2015;30:406–17.
Hargreaves R. Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. J Clin Psychiatry. 2002;63[Suppl 11]:18–24.
Prescribing information of EMEND for injection (fosaprepitant dimeglumine). http://www.merck.com/product/usa/pi_circulars/e/emend_iv/emend_iv_pi.pdf. Accessed 5 Oct 2016.
Langford P, Chrisp P. Fosaprepitant and aprepitant: an update of the evidence for their place in the prevention of chemotherapy-induced nausea and vomiting. Core Evid. 2010;5:77–90.
Kanda, Y. Free statistical software: EZR (Easy R) on R commander. http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html (2012). Accessed 1 Mar 2012.
Colon-Gonzalez F, Kraft WK. Pharmacokinetic evaluation of fosaprepitant dimeglumine. Expert Opin Drug Metab Toxicol. 2010;6:1277–86.
Cressman WA, Plostnieks J, Johnson PC. Absorption, metabolism and excretion of droperidol by human subjects following intramuscular and intravenous administration. Anesthesiology. 1973;38:363–9.
Acknowledgments
The authors express their sincere gratitude to all anesthesiologists, neurosurgeons, nurses, and pharmacists who supported this study. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Funding source
This study was performed at Nara Medical University Hospital and was solely supported by the Department of Anesthesiology, Nara Medical University.
About this article
Cite this article
Atsuta, J., Inoue, S., Tanaka, Y. et al. Fosaprepitant versus droperidol for prevention of PONV in craniotomy: a randomized double-blind study. J Anesth 31, 82–88 (2017). https://doi.org/10.1007/s00540-016-2267-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-016-2267-1