Abstract
Purpose
The pathological mechanisms of critical illness polyneuropathy (CIP), an acute neuromuscular disorder, remain unknown. In this study, we evaluated nerve and vascular properties that might account for electrophysiological abnormalities, including reduced nerve conduction amplitude, in the early phase of CIP.
Methods
Rats were administered intravenous saline (C-group; n = 31) or lipopolysaccharide (3 mg/kg/day; L-group; n = 30) for 48 h. Subsequently, tracheotomy was performed and sciatic nerves exposed bilaterally. A catheter was inserted into the left internal carotid artery to measure the mean arterial pressure (MAP). Nerve conduction velocity (NCV), nerve blood flow (NBF), evoked amplitudes, chronaxie, rheobase, and the absolute refractory period (ARP) were measured from the sciatic nerves. Degeneration, myelination, and neutrophil infiltration were examined in the sciatic nerves using histology and electron microscopy.
Results
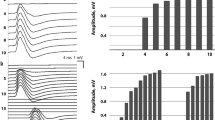
The NBF (C-group 25 ± 3 ml/100 g/min, L-group 13 ± 3 ml/100 g/min, p < 0.001) was lower in the L-group, but the MAP was similar between groups (C-group 119 ± 17 mmHg, L-group 115 ± 18 mmHg, p = 0.773). LPS also caused a severe reduction in amplitude (C-group 0.9 ± 0.2 mV, L-group 0.2 ± 0.1 mV, p < 0.001), while latency and NCV were not affected. Of note, response amplitudes partially recovered with an increase in stimulus intensity. LPS treatment increased the rheobase and decreased the chronaxie (rheobase: C vs L-group; 0.35 ± 0.07 vs 1.29 ± 0.66 mA, p < 0.001; chronaxie 171 ± 24 vs 42 ± 20 µs, p < 0.001), while ARP was unchanged. No primary axonal degeneration or inflammatory infiltration was observed.
Conclusions
Our findings suggest that primary electrophysiological deterioration is due to threshold alterations rather than morphological alterations after 48 h of LPS treatment.





Similar content being viewed by others
References
Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47:1223–31.
Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–91.
Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–41.
Griffiths RD, Jones C. Recovery from intensive care. BMJ. 1999;319:427–9.
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93.
Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, Sibbald WA. Critical illness polyneuropathy. A complication of sepsis and multiple organ failure. Brain. 1987;110:819–41.
Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol. 2003;106:75–82.
Sasano J, Hino H, Uchida K, Tateda T, Kazama A, Tadokoro M. The changes of microcirculatory dynamics in peripheral nerves in acute septic rat model (in Japanese with English abstract). St. Marianna Med J. 2006;34:229–39.
Witt NJ, Zochodne DW, Bolton CF, Grand’Maison F, Wells G, Young GB, Sibbald WJ. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–84.
Cankayali I, Dogan YH, Solak I, Demirag K, Eris O, Demirgoren S, Moral AR. Neuromuscular deterioration in the early stage of sepsis in rats. Crit Care. 2007;11:R1.
Rich MM, Kraner SD, Barch RL. Altered gene expression in steroid-treated denervated muscle. Neuro Dis. 1999;6:515–22.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC. Assessment of clinical criteria for sepsis: for the third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–74.
Iba T, Yagi Y, Kidokoro A, Tsukata M, Kondo T, Maruyama T. Evaluation of a rat model for disseminated intravascular coagulation developed by infusion of lipopolysaccharide (in Japanese with English abstract). JJAAM. 1997;8:103–10.
Shibayama Y, Asaka S, Urano T, Araki M, Oda K. Role of neutrophils and platelets in the pathogenesis of focal hepatocellular necrosis in endotoxaemia. Exp Toxicol Pathol. 1995;47:35–9.
Zifko UA, Zipko HT, Bolton CF. Clinical and electrophysiological findings in critical illness polyneuropathy. J Neurol Sci. 1998;159:186–93.
Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathy in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22:849–55.
Ashley Z, Sutherland H, Lanmuller H, Unger E, Li F, Mayr W, Kern H, Jarvis JC, Salmons S. Determination of the chronaxie and rheobase of denervated limb muscles in conscious rabbits. Artif Organs. 2005;29:212–5.
Irnich W. The chronaxie time and its practical importance. Pacing Clin Electrophysiol. 1980;3:292–301.
Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol. 2003;547:555–66.
Kraner SD, Novak KR, Wang Q, Peng J, Rich MM. Altered sodium channel-protein associations in critical illness myopathy. Skelet Muscle. 2012;30:17.
Myers RR, Yamamoto T, Yaksh TL, Powell HC. The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology. 1993;78:308–16.
Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest. 2009;119:1150–8.
Yang JS, Sladky JT, Kallen RG, Barchi RL. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron. 1991;7:421–7.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82.
Acknowledgments
We thank Prof. Tatsuo Akema for his helpful suggestions for the nerve conduction studies, and Dr. Masatomo Doi, Prof. Masayuki Takagi, and Prof. Mamoru Tadokoro for suggestions for pathology diagnosis using histology and electron microscopy. This research was supported by JSPS KAKENHI Grant Number 20592130.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Miura, A., Hino, H., Uchida, K. et al. Peripheral nerve conduction abnormalities precede morphological alterations in an experimental rat model of sepsis. J Anesth 30, 961–969 (2016). https://doi.org/10.1007/s00540-016-2247-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-016-2247-5