Abstract
Background
Hepatic arterial infusion chemotherapy (HAIC) with fluorouracil, leucovorin, and oxaliplatin (FOLFOX), lenvatinib and programmed death receptor-1 signaling inhibitors (PD1s) all alone have been proven effective in treating advanced hepatocellular carcinoma (HCC), yet the efficacy and safety of the tri-combination therapy in treating HCC patients with portal vein tumor thrombosis (PVTT) remains unknown.
Methods
In this retrospective study, HCC patients with PVTT received either induction therapy of HAIC and lenvatinib plus PD1s in the initial period of treatment and then dual maintenance therapy of lenvatinib and PD1s (HAIC-Len-PD1) or continuous lenvatinib combined with PD1s (Len-PD1).
Results
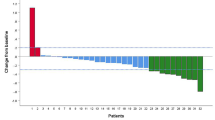
In total, 53 and 89 patients were enrolled into the Len-PD1 group and HAIC-Len-PD1 group, respectively. The median overall survival times were 13.8 months in the Len-PD1 group and 26.3 months in the HAIC-Len-PD1 group (hazard ratio (HR) = 0.43, P < 0.001). The median progression-free survival (PFS) time was significantly longer in the HAIC-Len-PD1 group than in the Len-PD1 group (11.5 months versus 5.5 months, HR = 0.43, P < 0.001). Induction therapy showed an objective response rate (ORR) 3 times higher than lenvatinib combined with PD1s therapy (61.8% versus 20.8%, P < 0.001), and exhibited inspiring intra- and extra-hepatic tumor control ability. Induction therapy led to more adverse events than lenvatinib combined with PD1s therapy, most of which were tolerable and controllable.
Conclusion
The induction therapy of FOLFOX-HAIC and lenvatinib plus PD1s is an effective and safe treatment for HCC patients with PVTT. The concept of induction therapy could be applied to other local–regional treatments and drugs combinations in HCC management.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;6:394–424.
Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394:1145–58.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721–30.
Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–93.
Su GL, Altayar O, O’Shea R, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 2022;162:920–34.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–70.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10:181–223.
Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9:682–720.
Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60–9.
Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. 2022;40:468–80.
Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III Trial. J Clin Oncol. 2022;40:150–60.
Kosaka Y, Kimura T, Kawaoka T, et al. Hepatic arterial infusion chemotherapy combined with radiation therapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk or bilobar of the portal vein. Liver Cancer. 2021;10:151–60.
He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–60.
European Association for the Study of the Liver. Electronic address eee, European association for the study of the L: EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis treatment and follow-up. Ann Oncol. 2018;29:iv238–55.
Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label. Multicenter Controlled Study J Clin Oncol. 2019;37:2141–51.
Altman DG, De Stavola BL, Love SB, et al. Review of survival analyses published in cancer journals. Br J Cancer. 1995;72:511–8.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80.
Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 edition). Liver Cancer. 2020;9:28–40.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379.
Bejjani AC, Finn RS. Hepatocellular carcinoma: pick the winner-tyrosine kinase inhibitor versus immuno-oncology agent-based combinations. J Clin Oncol. 2022;40:2763–73.
Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo B, Ren Z, Cheng A, Galle PR, Kaneko S, Kumada H, Wang A, Mody K, Dubrovsky L, Siegel AB, Llovet: Primary results from the phase III LEAP-002 study. Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annal Oncol. 2022;33:S808–69.
Emens LA, Ascierto PA, Darcy PK, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29.
Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III randomized clinical trial (LAUNCH). J Clin Oncol. 2022;41:117–27.
Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303:455–64.
Chabanon RM, Rouanne M, Lord CJ, et al. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17.
Guo J, Yu Z, Sun D, et al. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol Cancer. 2021;20:10.
Herrera FG, Irving M, Kandalaft LE, et al. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019;20:e417–33.
Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–65.
Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–71.
Huang J, Huang W, Zhan M, et al. Drug-eluting bead transarterial chemoembolization combined with FOLFOX-based hepatic arterial infusion chemotherapy for large or huge hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1445–58.
Funding
This work is funded by the National Natural Science Foundation of China (No: 81874070).
Author information
Authors and Affiliations
Contributions
YF: conceptualization, methodology, software, formal analysis, writing—original draft; WP: methodology, software, formal analysis; WZ: conceptualization, software, resources, data curation; ZY: conceptualization, software, resources, data curation; ZH: resources, investigation; YP: resources, investigation; DH: resources, investigation; JC: resources, investigation; JW: visualization, supervision; ZZ: supervision, data curation; LX: supervision, data curation; MC: conceptualization, funding acquisition, project administration, supervision; YZ: conceptualization, methodology, project administration, supervision, writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2023_1976_MOESM1_ESM.docx
Supplementary file1 Fig. S1. Flowchart for patient inclusion. Abbreviations: HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; TKIs, multi-tyrosine kinase inhibitors; ICIs, immune checkpoint inhibitors; HAIC, hepatic arterial infusion chemotherapy (DOCX 27 KB)
535_2023_1976_MOESM2_ESM.docx
Supplementary file2 Fig. S2. The median follow-up times of the two groups calculated by the reversed Kaplan–Meier method (DOCX 27 KB)
535_2023_1976_MOESM3_ESM.tif
Supplementary file3 Fig. S3. The detailed treatment courses of the two groups of patients and the second-line treatments after initial tumor progression. The number after each bar represents the total number of patients who received the corresponding treatment in the two groups (left: Len-PD1 group, right: HAIC-Len-PD1 group). Abbreviations: HAIC, hepatic arterial infusion chemotherapy; Len, lenvatinib; PD1s, programmed death receptor-1 signaling inhibitors; TACE, transarterial chemoembolization; TKIs, multi-tyrosine kinase inhibitors; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy (TIF 1594 KB)
535_2023_1976_MOESM4_ESM.tif
Supplementary file4 Fig S4. The progression-free survival of the two groups of patients receiving second-line treatments after initial tumor progression (a), and the efficacy of HAIC combined with lenvatinib plus PD1s versus other treatments as second-line therapy in the Len-PD1 group (b). Abbreviations: PFS, progression-free survival; HAIC, hepatic arterial infusion chemotherapy; Len, lenvatinib; PD1s, programmed death receptor-1 signaling inhibitors (TIF 1068 KB)
535_2023_1976_MOESM5_ESM.tif
Supplementary file5 Fig. S5. Subgroup analysis for the overall survival and progression-free survival of the two groups of patients after propensity score matching. Kaplan–Meier curves of (a-c) overall survival and (d-f) progression-free survival for patients with different portal vein tumor thrombosis stages. Kaplan–Meier curves of (g and h) overall survival and (i and j) progression-free survival for patients with or without extra-hepatic metastasis at the beginning of the treatments. Abbreviations: OS, overall survival; PFS, progression-free survival; Vp, Japan's portal vein invasion classification (TIF 3226 KB)
535_2023_1976_MOESM6_ESM.tif
Supplementary file6 Fig. S6. Comparison of efficacy regarding progression-free survival (a, c, e, and g) and overall survival (b, d, f, and h) among different PD1 drugs in the two groups both before and after propensity score matching. Abbreviations: PFS, progression-free survival; OS, overall survival; HAIC, hepatic arterial infusion chemotherapy; Len, lenvatinib; PD1s, programmed death receptor-1 signaling inhibitors; Pembro, Pembrolizumab; Sinti, Sintilimab; Tori, Toripalimab; Camre, Camrelizumab, Tisle, Tislelizumab (TIF 3445 KB)
535_2023_1976_MOESM7_ESM.tif
Supplementary file7 Fig. S7. The Sankey diagram of the dynamic changes of ALBI grades in the two groups of patients. The number above each bar represents the corresponding number of patients. Abbreviations: HAIC, hepatic arterial infusion chemotherapy; Len, lenvatinib; PD1s, programmed death receptor-1 signaling inhibitors; ALBI, Albumin-Bilirubin grade (TIF 1248 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Y., Peng, W., Zhang, W. et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol 58, 413–424 (2023). https://doi.org/10.1007/s00535-023-01976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-01976-x