Abstract
Background
Recently, Helicobacter pylori (HP)-uninfected gastric mucosal cancer has been reported; however, the clinicopathological and molecular features of HP-uninfected gastric cancer have not been elucidated.
Methods
We evaluated the clinicopathological, immunohistochemical, and genetic alterations in HP-uninfected early gastric adenocarcinoma using next-generation sequencing (NGS).
Results
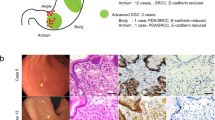
Among 968 primary early gastric carcinomas, 64 (6.6%) were HP-uninfected gastric adenocarcinoma and were pathologically classified as gastric adenocarcinoma of fundic-gland type (GA-FG, n = 39), differentiated gastric adenocarcinoma (DGA, n = 16), and signet-ring cell carcinoma (SRCC, n = 9). Based on the expression profile of the mucin core protein, DGAs were classified into a gastrointestinal phenotype showing either MUC5AC or MUC6 expression and MUC2 or CD10 expression simultaneously (n = 5), and a gastric phenotype (n = 11) showing either MUC5AC or MUC6 expression. All DGAs with a gastrointestinal phenotype shared similar endoscopic characteristics, such as reddish depressed lesions in the antrum. In contrast, DGAs with a gastric phenotype exhibited several distinct endoscopic features, including a raspberry-shaped appearance and whitish flat-elevated appearance; the former expressed only MUC5AC and the latter exhibited co-expression of MUC5AC and MUC6. Among 16 HP-uninfected DGAs, seven were subjected to NGS. APC was recurrently mutated in DGA (42.9%) and was enriched in DGAs with a gastrointestinal phenotype (75%).
Conclusions
Overall, HP-uninfected gastric adenocarcinomas showed distinct clinicopathologic and endoscopic characteristics. Furthermore, HP-uninfected DGAs, especially those with a gastrointestinal phenotype, may be characterized by recurrent APC mutations.




Similar content being viewed by others
References
N Uemura S Okamoto S Yamamoto 2001 Helicobacter pylori infection and the development of gastric cancer N Engl J Med 345 784 789
T Matsuo M Ito S Takata 2011 Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese Helicobacter 16 415 419
Y Yamamoto J Fujisaki M Omae 2015 Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings Dig Endosc 27 551 561
A Yamada M Kaise N Inoshita 2018 Characterization of helicobacter pylori-naive early gastric cancers Digestion 98 127 134
Y Horiuchi J Fujisaki N Ishizuka 2017 Study on clinical factors involved in helicobacter pylori-uninfected undifferentiated-type early gastric cancer Digestion 96 213 219
T Mizutani H Araki C Saigo 2020 Endoscopic and pathological characteristics of helicobacter pylori infection-negative early gastric cancer Dig Dis 38 6 1 10
H Ueyama T Yao Y Nakashima 2010 Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma Am J Surg Pathol 34 609 619
N Yorita M Ito T Boda 2019 Potential of helicobacter pylori-uninfected signet ring cell carcinoma to invade the submucosal layer J Gastroenterol Hepatol 34 1955 1962
Board WCoTE. WHO Classification of Tumours, 5th ed, Vol 1 Digestive system tumours. Lyon. IARC 2019.
H Ueyama T Yao Y Akazawa 2021 Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type J Gastroenterol 56 814 828
H Ueyama K Matsumoto A Nagahara 2014 Gastric adenocarcinoma of the fundic gland type (chief cell predominant type) Endoscopy 46 153 157
Y Ozaki H Suto T Nosaka 2015 A case of Helicobacter pylori-negative intramucosal well-differentiated gastric adenocarcinoma with intestinal phenotype Clin J Gastroenterol 8 18 21
S Kotani Y Miyaoka A Fujiwara 2016 Intestinal-type gastric adenocarcinoma without Helicobacter pylori infection successfully treated with endoscopic submucosal dissection Clin J Gastroenterol 9 228 232
S Yoshii Y Hayashi T Takehara 2017 Helicobacter pylori-negative early gastric adenocarcinoma with complete intestinal mucus phenotype mimicking verrucous gastritis Dig Endosc 29 235 236
K Shibagaki C Fukuyama H Mikami 2019 Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the helicobacter pylori -uninfected stomach Endosc Int Open 7 E784 E791
Y Isono Y Baba K Mukai 2018 Gastric adenocarcinoma coexisting with a reddish semipedunculated polyp arising from Helicobacter pylori-negative normal gastric mucosa: a report of two cases Clin J Gastroenterol 11 481 486
N Yatagai H Ueyama M Ikemura 2020 Clinicopathological and Endoscopic Features of Raspberry-Shaped Gastric Cancer in Helicobacter pylori-Uninfected Patients Digestion. 102 1 41 48
M Takita K Ohata R Inamoto 2021 Endoscopic and histological features of Helicobacter pylori-negative differentiated gastric adenocarcinoma arising in the antrum JGH Open 5 470 477
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, The 15th edn. Oct 2017.
T Yoshida T Yamaguchi S Maekawa 2019 Identification of early genetic changes in well-differentiated intramucosal gastric carcinoma by target deep sequencing Gastric Cancer 22 742 750
K Kimura T Takemoto 1969 An endoscopic recognition of the atrophic border and its significance in chronic gastritis Endoscopy 1 87 97
MA Benedict GY Lauwers D Jain 2018 Gastric adenocarcinoma of the fundic gland type: update and literature review Am J Clin Pathol 149 461 473
Y Akazawa T Saito T Hayashi 2018 Next-generation sequencing analysis for gastric adenocarcinoma with enteroblastic differentiation: emphasis on the relationship with hepatoid adenocarcinoma Hum Pathol 78 79 88
S Kishikawa T Hayashi T Saito 2020 Diffuse expression of MUC6 defines a distinct clinicopathological subset of pulmonary invasive mucinous adenocarcinoma Mod Pathol. 34 4 786 797
Y Kanda 2013 Investigation of the freely available easy-to-use software 'EZR' for medical statistics Bone Marrow Transpl 48 452 458
R Bonneville MA Krook EA Kautto 2017 Landscape of microsatellite instability across 39 cancer types JCO Precis Oncol https://doi.org/10.1200/PO.17.00073
L Ding MH Bailey E Porta-Pardo 2018 Perspective on oncogenic processes at the end of the beginning of cancer genomics Cell 173 305–20 e10
K Ellrott MH Bailey G Saksena 2018 Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines Cell Syst 6 271–81 e7
Q Gao WW Liang SM Foltz 2018 Driver fusions and their implications in the development and treatment of human cancers Cell Rep 23 227–38 e3
KA Hoadley C Yau T Hinoue 2018 Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer Cell 173 291–304 e6
J Liu T Lichtenberg KA Hoadley 2018 An integrated tcga pan-cancer clinical data resource to drive high-quality survival outcome analytics Cell 173 400–16 e11
GD Poore E Kopylova Q Zhu 2020 Microbiome analyses of blood and tissues suggest cancer diagnostic approach Nature 579 567 574
F Sanchez-Vega M Mina J Armenia 2018 Oncogenic signaling pathways in the cancer genome atlas Cell 173 321–37 e10
AM Taylor J Shih G Ha 2018 Genomic and functional approaches to understanding cancer aneuploidy Cancer Cell 33 676–89 e3
JKY Hooi WY Lai WK Ng 2017 Global prevalence of helicobacter pylori infection: systematic review and meta-analysis Gastroenterology 153 420 429
T Murakami H Mitomi T Yao 2017 Epigenetic regulation of Wnt/beta-catenin signal-associated genes in gastric neoplasia of the fundic gland (chief cell-predominant) type Pathol Int 67 147 155
Y Tajima T Murakami T Saito 2017 Distinct involvement of the sonic hedgehog signaling pathway in gastric adenocarcinoma of fundic gland type and conventional gastric adenocarcinoma Digestion 96 81 91
M Fassan M Simbolo E Bria 2014 High-throughput mutation profiling identifies novel molecular dysregulation in high-grade intraepithelial neoplasia and early gastric cancers Gastric Cancer 17 442 449
M Nikaido N Kakiuchi S Miyamoto 2021 Indolent feature of Helicobacter pylori-uninfected intramucosal signet ring cell carcinomas with CDH1 mutations Gastric Cancer 24 1102 1114
T Funakoshi S Miyamoto N Kakiuchi 2019 Genetic analysis of a case of helicobacter pylori-uninfected intramucosal gastric cancer in a family with hereditary diffuse gastric cancer Gastric Cancer 22 892 898
A Okano S Kato M Ohana 2017 Helicobacter pylori-negative gastric cancer: advanced-stage undifferentiated adenocarcinoma located in the pyloric gland area Clin J Gastroenterol 10 13 17
J Muraishi M Miyaoka K Imamura 2021 A case of gastric signet-ring cell carcinoma with a long-term retrospective follow-up of 17 years Clin J Gastroenterol 14 1337 1343
Cancer Genome Atlas Research N 2014 Comprehensive molecular characterization of gastric adenocarcinoma Nature. 513 202 9
AP Burke TS Yen KM Shekitka 1990 Lymphoepithelial carcinoma of the stomach with epstein-barr virus demonstrated by polymerase chain reaction Mod Pathol 3 377 380
N Zamcheck E Grable A Ley 1955 Occurrence of gastric cancer among patients with pernicious anemia at the Boston city hospital N Engl J Med 252 1103 1110
K Hizawa M Iida T Yao 1997 Juvenile polyposis of the stomach: clinicopathological features and its malignant potential J Clin Pathol 50 771 774
GN Mankaney M Cruise S Sarvepalli 2020 Surveillance for pathology associated with cancer on endoscopy (SPACE) criteria to identify high-risk gastric polyps in familial adenomatous polyposis Gastrointest Endosc 92 3 755 762
Acknowledgements
This work was performed in part at the Intractable Disease Research Center, Juntendo University. The authors thank Noriko Sasahara for the technical assistance. This work was financially supported in part by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports, and Culture (JSPS KAKENHI Grant Numbers JP 19K17412 and 20K08393), Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
YA, TH, and HU. conceived and designed the study and wrote, edited, and reviewed the manuscript. YA, HU, RU, DA, SO, NS, AI, NY, HK, TT, KM, KU, KM, DA, MH, and AN. provided clinical and endoscopic information. TH, TS, and TY. provided histological and immunohistochemical information. HU, TY, and AN. supervised the study. TH. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Juntendo University School of Medicine (#2016107). The patients were not required to provide consent for participation in the study, but we offered opt-out option, which allows patients to express a choice to object to their confidential patient information being used in this research. Individuals cannot be identified from the data presented here.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2022_1906_MOESM1_ESM.tif
Supplementary file 1 (TIF 1330 KB) Classification of mucin phenotypes using immunohistochemical staining. The gastric phenotype was positive only for MUC5AC and/or MUC6, whereas the intestinal phenotype was positive only for MUC2 and/or CD10. Lesions showing both phenotypic expressions were classified into the gastrointestinal phenotype, and those with no phenotypic expression were classified into the unclassified phenotype.
535_2022_1906_MOESM2_ESM.tif
Supplementary file2 (TIF 7147 KB) Gastric adenocarcinoma of the fundic-gland type (GA-FG). a. White light imaging. The flatly elevated lesion with submucosal tumor shape was located at the greater curvature of the cardia and had a whitish color and dilated vessels with branching architecture. The background mucosa had no atrophic change. b. Magnifying endoscopy with narrow-band imaging. No irregularity was observed in the vessels and on the surface structure. c. Hematoxylin and eosin (HE) staining (×40). Gastric adenocarcinoma resembling fundic-gland cells was located from beneath the surface to the deep area with an invasion depth of 600 μm. The surface of the lesion was covered with normal foveolar epithelium, whereas the deep area of the tumor showed irregular branching and dilatation. d. HE staining (×200). The tumor cells were mainly composed of highly differentiated columnar cells mimicking fundic-gland cells, predominantly chief cells, with pale gray blue, basophilic cytoplasm and mildly enlarged nuclei. e-l. Immunohistochemistry (×100). e. pepsinogen I: positive, f. H+/K+ ATPase: focally positive, g. MUC5AC: negative, h. MUC6: negative, i. MUC2: negative, j. CD10: negative, k. p53 overexpression: negative, l. Ki-67 labeling Index=1%.
535_2022_1906_MOESM3_ESM.tif
Supplementary file3 (TIF 2778 KB) a, b. White light and narrow-band imaging. The whitish depressed lesion was located at the greater curvature of the lower third of the stomach. c. Magnifying endoscopy with narrow-band imaging. The tumor showed regular vessels and regular surface structure with no demarcation line and had no cancerous findings. d. HE (×200). Signet ring-cells were located from beneath the surface to the middle layer of the mucosa. The surface of the lesion was covered with normal foveolar epithelium. e-j. Immunohistochemistry (×200). e. MUC5AC: positive, f. MUC6: negative, g. MUC2: negative, h CD10: negative, i. p53 overexpression: negative, j. Ki-67 labeling Index=5%
535_2022_1906_MOESM4_ESM.tif
Supplementary file4 (TIF 956 KB) APC mutation rate between Helicobacter pylori-uninfected differentiated gastric adenocarcinoma and conventional gastric adenocarcinoma. Compared to The Cancer Genome Atlas database, differentiated gastric adenocarcinoma (DGA) showed a significantly higher APC mutation rate than conventional gastric adenocarcinoma (CGA). *P < 0.05.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akazawa, Y., Ueyama, H., Hayashi, T. et al. Clinicopathological and molecular characterization of early gastric adenocarcinoma in Helicobacter pylori-uninfected patients: emphasis on differentiated gastric adenocarcinoma. J Gastroenterol 57, 725–734 (2022). https://doi.org/10.1007/s00535-022-01906-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-022-01906-3