Abstract
Background
Various serologic markers such as anti-glycoprotein 2 antibodies and anti-Saccharomyces cerevisiae antibodies have been reported to be diagnostically useful in Crohn’s disease. Mitsuyama et al. reported that antibodies to Crohn’s disease peptide 353, a newly proposed serologic marker, were more useful in Japanese adults than anti-Saccharomyces. We addressed the same issue in Japanese children and adolescents.
Methods
Prospectively enrolled subjects under 17 years old assessed and treated at 12 pediatric centers in Japan included groups with Crohn’s disease, ulcerative colitis, other intestinal diseases, or good health. The 3 serum markers were analyzed by enzyme-linked immunosorbent assays.
Results
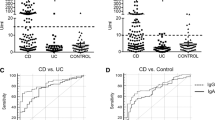
Enrolled subjects, numbering 367, included 120 with Crohn’s disease, 148 with ulcerative colitis, 56 with other intestinal diseases, and 43 healthy subjects. In Crohn’s disease, anti-Crohn’s disease peptide 353, anti-glycoprotein 2, and anti-Saccharomyces concentrations (median, 2.25, 3.0, and 8.9 U/mL) were significantly greater than in ulcerative colitis (1.1, 1.9, and 3.4; all P < 0.001), other intestinal diseases (1.1, 1.85, and 2.95; all P < 0.001), and healthy controls (1.1, 1.7, and 2.8; all P < 0.001), respectively. At 95% specificity, sensitivity of anti-Crohn’s disease peptide (45.0%) was significantly higher than for anti-glycoprotein 2 (30.8%; P < 0.05) or anti-Saccharomyces (26.7%; P < 0.01).
Conclusions
Anti-Crohn’s disease peptide 353 proved more useful for diagnosis of Crohn’s disease in Japanese children than the other 2 markers. To our knowledge, this is the first pediatric report to that effect.


Similar content being viewed by others
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- IBDU:
-
Inflammatory bowel disease unclassified
- ANCA:
-
Antineutrophil cytoplasmic antibodies
- ASCA:
-
Anti-Saccharomyces cerevisiae antibodies
- GP2:
-
Anti-glycoprotein 2 antibodies
- ACP353:
-
Antibodies to Crohn’s disease peptide 353
- MBL:
-
Medical & Biological Laboratories
- IC:
-
Intestinal disease control
- XIAP:
-
X-linked inhibitor of apoptosis
- IBS:
-
Irritable bowel syndrome
- HC:
-
Healthy control
- PCDAI:
-
Pediatric Crohn’s Disease Activity Index
- PUCAI:
-
Pediatric Ulcerative Colitis Activity Index
- ELISA:
-
Enzyme-linked immunosorbent assay
- IgG:
-
Immunoglobulin G
- PBS:
-
Phosphate-buffered saline
- ROC:
-
Receiver operating characteristic
References
Mitsuyama K, Niwa M, Takedatsu H, et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol. 2016;22:1304–10.
Mitsuyama K, Niwa M, Masuda J, et al. Possible diagnostic role of antibodies to Crohn's disease peptide (ACP): results of a multicenter study in a Japanese cohort. J Gastroenterol. 2014;49:683–91.
Takedatsu H, Mitsuyama K, Fukunaga S, et al. Diagnostic and clinical role of serum proteinase 3 antineutrophil cytoplasmic antibodies in inflammatory bowel disease. J Gastroenterol Hepatol. 2018. https://doi.org/10.1111/jgh.14140.
Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–91.
Saxon A, Shanahan F, Landers C, et al. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–10.
Horn MP, Peter AM, Righini Grunder F, et al. PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn's disease. PLoS One. 2018;13:e0208974.
Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105–6.
Gironella M, Iovanna JL, Sans M, et al. Anti-inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut. 2005;54:1244–53.
Roggenbuck D, Hausdorf G, Martinez-Gamboa L, et al. Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn's disease. Gut. 2009;58:1620–8.
Rober N, Noss L, Goihl A, et al. Autoantibodies against glycoprotein 2 isoforms in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1624–36.
Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn's disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–99.
Wei B, Huang T, Dalwadi H, et al. Pseudomonas fluorescens encodes the Crohn's disease-associated I2 sequence and T-cell superantigen. Infect Immun. 2002;70:6567–75.
Sitaraman SV, Klapproth JM, Moore DA 3rd, et al. Elevated flagellin-specific immunoglobulins in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G403–G406406.
Hisabe T, Matsui T, Sakurai T, et al. Anti-Saccharomyces cerevisiae antibodies in Japanese patients with inflammatory bowel disease: diagnostic accuracy and clinical value. J Gastroenterol. 2003;38:121–6.
Mitsuyama K, Niwa M, Masuda J, et al. Isolation and characterization of a novel short peptide associated with Crohn's disease. Clin Exp Immunol. 2011;166:72–9.
El-Matary W, Dupuis K, Sokoro A. Anti-Saccharomyces cerevisiae antibody titres correlate well with disease activity in children with Crohn's disease. Acta Paediatr. 2015;104:827–30.
Russell RK, Ip B, Aldhous MC, et al. Anti-Saccharomyces cerevisiae antibodies status is associated with oral involvement and disease severity in Crohn disease. J Pediatr Gastroenterol Nutr. 2009;48:161–7.
Levine A, Koletzko S, Turner D, et al. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806.
Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21.
Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–47.
Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–32.
Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–30.
Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–22.
Ruemmele FM, Targan SR, Levy G, et al. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–9.
Saito H, Fukuda Y, Katsuragi K, et al. Isolation of peptides useful for differential diagnosis of Crohn's disease and ulcerative colitis. Gut. 2003;52:535–40.
Acknowledgements
The authors thank all participating patients, their families, and physicians for collaborating in data collection.
Funding
None.
Author information
Authors and Affiliations
Contributions
Concept and design of study, TM and KM; acquisition of serum samples and data, TM, KA, TaK, RN, HT, TA, NA, ToK, KH, TS, MT, YE, YT, KK, JI, YY, and KM; serum antibody assays, SK; analysis and interpretation of data, TM, HO, TaK, and KM; drafting of the article, TM; refining the draft, TM, TaK, YY, and KM; critical review and approval of the draft, all authors.
Corresponding author
Ethics declarations
Conflict of interest
Shunsuke Kurei is an employee of Medical & Biological Laboratories, a collaborating company where the serum antibody assays were performed in a blinded manner without interpretation of data. Toagosei Co., Ltd. and Kurume University have applied to register a patent concerning the ACP353 test. The title of the invention is: Crohn's disease antibody epitope peptide and reagent for testing Crohn's disease. Applicable registration numbers are JP4597132; EP 1780215; US 7,985,831; KR 10–1159626; and ZL200910159881.0(CN).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

535_2019_1661_MOESM2_ESM.jpg
Supplementary Figure 1. ROC curves for ACP353, GP2, and ASCA among IBD, IBS, and infectious enterocolitis. Areas under receiver operating characteristic (ROC) curves for antibodies to Crohn’s disease peptide 353 (ACP353)/anti-glycoprotein 2 antibodies (GP2)/anti-Saccharomyces cerevisiae antibodies (ASCA) in distinguishing inflammatory bowel disease (IBD) including Crohn’s disease (CD), ulcerative colitis (UC), IBD-unclassified, Behçet's disease, and X-linked inhibitor of apoptosis deficiency from irritable bowel syndrome (IBS) [A]; IBD from infectious enterocolitis [B]; and CD from UC [C] were 0.66/0.52/0.61, 0.53/0.67/0.68, and 0.78/0.72/0.72, respectively (JPG 3485 kb)
Rights and permissions
About this article
Cite this article
Mizuochi, T., Arai, K., Kudo, T. et al. Antibodies to Crohn’s disease peptide 353 as a diagnostic marker for pediatric Crohn’s disease: a prospective multicenter study in Japan. J Gastroenterol 55, 515–522 (2020). https://doi.org/10.1007/s00535-019-01661-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01661-y