Abstract
Purpose
The Multinational Association of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) Patient Antiemetic Guideline Committee aimed to (1) adapt the updated evidence-based, clinical guidelines to patient-centered antiemetic guidelines and (2) develop patient education materials and statements.
Methods
The MASCC 2023 Patient Antiemetic Guidelines were created and reviewed by antiemetic experts and patient advocates by incorporating the 2023 MASCC/ESMO antiemetic guidelines into patient-friendly language. Patient Education Statements were developed based on current literature and by utilizing an expert modified Delphi consensus (≥ 75% agreement). Patient advocate/focus group input and patient survey results were further integrated into Patient-Centered Antiemetic Guidelines and Education Statements.
Results
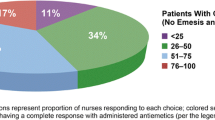
Patient-Centered Antiemetic Guidelines were created using patient-friendly language and visual slides. Patient-friendly language was also utilized to communicate the Educational Statements. Key content categories identified for the Educational Statements included the following: nausea/vomiting definitions, causes, risk factors, categories, complications, accompanying symptoms, prophylactic antiemetic treatment, general management, when to call/what to ask the healthcare team, what caregivers can do, and available resources. All identified content met the ≥ 75% expert agreement threshold. Fifteen (15) items demonstrated 100% agreement, 11 items achieved ≥ 90% agreement, and three content items demonstrated 80 ~ 82% agreement.
Conclusions
The inaugural MASCC 2023 Patient Antiemetic Guidelines can help patients and caregivers understand the prevention of nausea and vomiting related to their cancer treatment. Educational Statements provide further patient information. Educating patients on how to utilize guideline antiemetics and the education statements can contribute improvements in the control of anticancer treatment-related nausea and vomiting.


Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Navari RM, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374(14):1356–1367. https://doi.org/10.1056/NEJMra1515442
Aapro M (2018) CINV: still troubling patients after all these years. Support Care Cancer 26(Suppl 1):5–9. https://doi.org/10.1007/s00520-018-4131-3
Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, Jordan K, Young A, Aapro M (2017) The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol 28(6):1260–1267. https://doi.org/10.1093/annonc/mdx100
Razvi Y, Chan S, McFarlane T, McKenzie E, Zaki P, DeAngelis C, Pidduck W, Bushehri A, Chow E, Jerzak KJ (2019) ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer 27(1):87–95. https://doi.org/10.1007/s00520-018-4464-y
Armstrong MJ, Mullins CD, Gronseth GS, Gagliardi AR (2018) Impact of patient involvement on clinical practice guideline development: a parallel group study. Implement Sci 13(1):55. https://doi.org/10.1186/s13012-018-0745-6
Addario B, Geissler J, Horn MK, Krebs LU, Maskens D, Oliver K, Plate A, Schwartz E, Willmarth N (2020) Including the patient voice in the development and implementation of patient-reported outcomes in cancer clinical trials. Health Expect 23(1):41–51. https://doi.org/10.1111/hex.12997
Paterick TE, Patel N, Tajik AJ, Chandrasekaran K (2017) Improving health outcomes through patient education and partnerships with patients. Proc (Bayl Univ Med Cent) 30(1):112–113. https://doi.org/10.1080/08998280.2017.11929552
Molassiotis A, Zhao IY, Crichton M, Olver I, Fleury M, Giusti R, Scotte F, Affronti ML (2023) Effects of food-based interventions in the management of chemoradiotherapy-induced nausea and vomiting: a systematic review. Support Care Cancer 31(7):413. https://doi.org/10.1007/s00520-023-07879-0
Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, Rittenberg C, Gralla RJ (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage 34(2):148–159. https://doi.org/10.1016/j.jpainsymman.2006.10.018
Salsman JM, Grunberg SM, Beaumont JL, Rogers M, Paul D, Clayman ML, Cella D (2012) Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Canc Netw 10(2):149–157. https://doi.org/10.6004/jnccn.2012.0018
Acknowledgements
We appreciate Ruxandra Nedu for her assistance in conducting the expert consensus survey. We also thank Yun Young Choi, Bomi Hong, and So Jung Kim who helped scope websites for Patient Antiemetic Education.
Funding
Mary Lou Affronti has received research support from TerSera who provided rolapitant and funding for an investigator-initiated study (Pro00076418). TerSera’s role for this investigator-initiated study was to review/approve the protocol and manuscripts. No protocol writing or data analysis was conducted by TerSera.
Author information
Authors and Affiliations
Contributions
All authors contributed. The first draft of the manuscript was written by M.L. Affronti and all authors commented on previous versions of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This did not require an ethical approval or consent.
Consent for publication
Not applicable. No images or identifying information of human subjects were included in the publication.
Competing interests
Dr. Mary L. Affronti received funding from TeSera to conduct an investigator-initiated Phase II Study, is member of the MASCC Antiemetic study group and on the 2023 Antiemetic Guideline Consensus Committee (Chairs the Patient Antiemetic Guideline committee; Member of Integrative/Nonpharmological Antiemetic Guideline committee) without financial compensation. Dr. Affronti is on the 2023 Society for Neuro-Oncology serving as Board Directors and receives no financial compensation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Affronti, M.L., Lee, J., Molassiotis, A. et al. MASCC 2023 Patient-Centered Antiemetic Guidelines and Education Statements: an evidence-based and consensus resource for patients. Support Care Cancer 32, 335 (2024). https://doi.org/10.1007/s00520-024-08543-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08543-x