Abstract
Purpose
This review aimed to assess the measurement and reporting of time toxicity (i.e., time spent receiving care) within prospective oncologic studies.
Methods
On July 23, 2023, PubMed, Scopus, and Embase were queried for prospective or randomized controlled trials (RCT) from 1984 to 2023 that reported time toxicity as a primary or secondary outcome for oncologic treatments or interventions. Secondary analyses of RCTs were included if they reported time toxicity. The included studies were then evaluated for how they reported and defined time toxicity.
Results
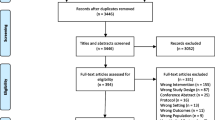
The initial query identified 883 records, with 10 studies (3 RCTs, 2 prospective cohort studies, and 5 secondary analyses of RCTs) meeting the final inclusion criteria. Treatment interventions included surgery (n = 5), systemic therapies (n = 4), and specialized palliative care (n = 1). The metric “days alive and out of the hospital” was used by 80% (n = 4) of the surgical studies. Three of the surgical studies did not include time spent receiving ambulatory care within the calculation of time toxicity. “Time spent at home” was assessed by three studies (30%), each using different definitions. The five secondary analyses from RCTs used more comprehensive metrics that included time spent receiving both inpatient and ambulatory care.
Conclusions
Time toxicity is infrequently reported within oncologic clinical trials, with no standardized definition, metric, or methodology. Further research is needed to identify best practices in the measurement and reporting of time toxicity to develop strategies that can be implemented to reduce its burden on patients seeking cancer care.

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Daily K (2018) The toxicity of time. J Clin Oncol 36:300–301. https://doi.org/10.1200/JCO.2017.74.7907
Gupta A, Eisenhauer EA, Booth CM (2022) The time toxicity of cancer treatment. J Clin Oncol 40:1611–1615. https://doi.org/10.1200/JCO.21.02810
Hall ET, Sridhar D, Singhal S et al (2021) Perceptions of time spent pursuing cancer care among patients, caregivers, and oncology professionals. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 29:2493–2500. https://doi.org/10.1007/s00520-020-05763-9
van Roij J, Brom L, Youssef-El Soud M et al (2019) Social consequences of advanced cancer in patients and their informal caregivers: a qualitative study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 27:1187–1195. https://doi.org/10.1007/s00520-018-4437-1
Ariti CA, Cleland JGF, Pocock SJ et al (2011) Days alive and out of hospital and the patient journey in patients with heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J 162:900–906. https://doi.org/10.1016/j.ahj.2011.08.003
Mishra NK, Shuaib A, Lyden P et al (2011) Home time is extended in patients with ischemic stroke who receive thrombolytic therapy: a validation study of home time as an outcome measure. Stroke 42:1046–1050. https://doi.org/10.1161/STROKEAHA.110.601302
Taran S, Coiffard B, Huszti E et al (2023) Association of days alive and at home at day 90 after intensive care unit admission with long-term survival and functional status among mechanically ventilated patients. JAMA Netw Open 6:e233265. https://doi.org/10.1001/jamanetworkopen.2023.3265
Myles PS (2020) More than just morbidity and mortality - quality of recovery and long-term functional recovery after surgery. Anaesthesia 75(Suppl 1):e143–e150. https://doi.org/10.1111/anae.14786
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol 8:19–32. https://doi.org/10.1080/1364557032000119616
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/M18-0850
Morton RP, Stell PM (1984) Cytotoxic chemotherapy for patients with terminal squamous carcinoma–does it influence survival? Clin Otolaryngol Allied Sci 9:175–180. https://doi.org/10.1111/j.1365-2273.1984.tb01492.x
Nordly M, Skov Benthien K, Vadstrup ES et al (2019) Systematic fast-track transition from oncological treatment to dyadic specialized palliative home care: DOMUS - a randomized clinical trial. Palliat Med 33:135–149. https://doi.org/10.1177/0269216318811269
Panda N, Solsky I, Hawrusik B et al (2021) Smartphone global positioning system (GPS) data enhances recovery assessment after breast cancer surgery. Ann Surg Oncol 28:985–994. https://doi.org/10.1245/s10434-020-09004-5
Scott SI, Madsen AKØ, Rubek N et al (2021) Days alive and out of hospital after treatment for oropharyngeal squamous cell carcinoma with primary transoral robotic surgery or radiotherapy - a prospective cohort study. Acta Otolaryngol (Stockh) 141:193–196. https://doi.org/10.1080/00016489.2020.1836395
Catto JWF, Khetrapal P, Ricciardi F et al (2022) Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA 327:2092–2103. https://doi.org/10.1001/jama.2022.7393
Ritch CR, Cookson MS, Chang SS et al (2014) Impact of complications and hospital-free days on health related quality of life 1 year after radical cystectomy. J Urol 192:1360–1364. https://doi.org/10.1016/j.juro.2014.06.004
Prasad V, Olivier T, Chen EY, Haslam A (2022) Estimation of time cost of anti-cancer drugs approved based on comparisons to best supportive care: a cross sectional analysis. J Cancer Policy 34:100363. https://doi.org/10.1016/j.jcpo.2022.100363
Vejlgaard M, Maibom SL, Joensen UN et al (2022) Quality of life and secondary outcomes for open versus robot-assisted radical cystectomy: a double-blinded, randomised feasibility trial. World J Urol 40:1669–1677. https://doi.org/10.1007/s00345-022-04029-9
Gupta A, Hay AE, Crump M et al (2023) Contact days associated with cancer treatments in the CCTG LY.12 trial. Oncologist 28:799–803. https://doi.org/10.1093/oncolo/oyad128
Gupta A, O’Callaghan CJ, Zhu L et al (2023) Evaluating the time toxicity of cancer treatment in the CCTG CO.17 trial. JCO Oncol Pract 19:e859–e866. https://doi.org/10.1200/OP.22.00737
Fundytus A, Prasad V, Booth CM (2021) Has the current oncology value paradigm forgotten patients’ time?: Too little of a good thing. JAMA Oncol 7:1757–1758. https://doi.org/10.1001/jamaoncol.2021.3600
Morrison-Jones V, West M (2023) Post-operative care of the cancer patient: emphasis on functional recovery, rapid rescue, and survivorship. Curr Oncol Tor Ont 30:8575–8585. https://doi.org/10.3390/curroncol30090622
Auriemma CL, Taylor SP, Harhay MO et al (2021) Hospital-free days: a pragmatic and patient-centered outcome for trials among critically and seriously ill patients. Am J Respir Crit Care Med 204:902–909. https://doi.org/10.1164/rccm.202104-1063PP
Quinn TJ, Dawson J, Lees JS et al (2008) Time spent at home poststroke: “home-time” a meaningful and robust outcome measure for stroke trials. Stroke 39:231–233. https://doi.org/10.1161/STROKEAHA.107.493320
Gill TM, Gahbauer EA, Leo-Summers L et al (2019) Days spent at home in the last six months of life among community-living older persons. Am J Med 132:234–239. https://doi.org/10.1016/j.amjmed.2018.10.029
Engelhart S, Cheung M, Croxford R, Singh S (2021) Days spent at home before death from cancer for immigrants and long-term residents in Ontario, Canada. J Palliat Med 24:593–598. https://doi.org/10.1089/jpm.2020.0287
Burke LG, Orav EJ, Zheng J, Jha AK (2020) Healthy days at home: a novel population-based outcome measure. Healthc Amst Neth 8:100378. https://doi.org/10.1016/j.hjdsi.2019.100378
Onega T, Alford-Teaster J, Leggett C et al (2023) The interaction of rurality and rare cancers for travel time to cancer care. J Rural Health Off J Am Rural Health Assoc Natl Rural Health Care Assoc 39:426–433. https://doi.org/10.1111/jrh.12693
Segel JE, Lengerich EJ (2020) Rural-urban differences in the association between individual, facility, and clinical characteristics and travel time for cancer treatment. BMC Public Health 20:196. https://doi.org/10.1186/s12889-020-8282-z
Ray KN, Chari AV, Engberg J et al (2015) Opportunity costs of ambulatory medical care in the United States. Am J Manag Care 21:567–574
Sugalski JM, Kubal T, Mulkerin DL et al (2019) National comprehensive cancer network infusion efficiency workgroup study: optimizing patient flow in infusion centers. J Oncol Pract 15:e458–e466. https://doi.org/10.1200/JOP.18.00563
Bange EM, Doucette A, Gabriel PE et al (2020) Opportunity costs of receiving palliative chemotherapy for metastatic pancreatic ductal adenocarcinoma. JCO Oncol Pract 16:e678–e687. https://doi.org/10.1200/JOP.19.00328
Kagalwalla S, Tsai AK, George M, et al (2024) Consuming patients’ days: time spent on ambulatory appointments by people with cancer. The Oncologist oyae016. https://doi.org/10.1093/oncolo/oyae016
Gluck S, Andrawos A, Summers MJ et al (2022) The use of smartphone-derived location data to evaluate participation following critical illness: a pilot observational cohort study. Aust Crit Care Off J Confed Aust Crit Care Nurses 35:225–232. https://doi.org/10.1016/j.aucc.2021.05.007
Panda N, Solsky I, Huang EJ et al (2020) Using smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg 155:123–129. https://doi.org/10.1001/jamasurg.2019.4702
Uemura K, Iwamoto T, Hiromatsu M et al (2022) Objectively-measured out-of-home behavior and physical activity in rural older adults. Geriatr Nurs N Y N 47:18–22. https://doi.org/10.1016/j.gerinurse.2022.06.010
Pew Research Center Mobile Fact Sheet. In: Pew Res. Cent. Internet Sci. Tech. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed 27 Nov 2023
Eton DT, Ramalho de Oliveira D, Egginton JS et al (2012) Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas 3:39–49. https://doi.org/10.2147/PROM.S34681
Eton DT, Yost KJ, Lai J-S et al (2017) Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 26:489–503. https://doi.org/10.1007/s11136-016-1397-0
Bath NM, Sarna A, Palettas M et al (2023) Characterizing treatment burden during neoadjuvant therapy for patients with gastrointestinal cancer: a mixed methods analysis. J Surg Oncol 128:393–401. https://doi.org/10.1002/jso.27288
Adam R, Duncan L, Maclennan SJ, Locock L (2023) Treatment burden in survivors of prostate and colorectal cancers: a qualitative interview study. BMJ Open 13:e068997. https://doi.org/10.1136/bmjopen-2022-068997
El-Turk N, Chou MSH, Ting NCH et al (2021) Treatment burden experienced by patients with lung cancer. PLoS ONE 16:e0245492. https://doi.org/10.1371/journal.pone.0245492
Palmer S, Raftery J (1999) Economic notes: opportunity cost. BMJ 318:1551–1552. https://doi.org/10.1136/bmj.318.7197.1551
Spiller SA (2019) Opportunity cost neglect and consideration in the domain of time. Curr Opin Psychol 26:98–102. https://doi.org/10.1016/j.copsyc.2018.10.001
Lim S-A, Hao SB, Boyd BA et al (2022) Opportunity costs of surgical resection and perioperative chemotherapy for locoregional pancreatic adenocarcinoma. JCO Oncol Pract 18:302–309. https://doi.org/10.1200/OP.21.00311
Krouse RS, Anderson GL, Arnold KB et al (2023) Surgical versus non-surgical management for patients with malignant bowel obstruction (S1316): a pragmatic comparative effectiveness trial. Lancet Gastroenterol Hepatol 8:908–918. https://doi.org/10.1016/S2468-1253(23)00191-7
Nindra U, Shivasabesan G, Childs S et al (2023) Time toxicity associated with early phase clinical trial participation. ESMO Open 8:102046. https://doi.org/10.1016/j.esmoop.2023.102046
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by P.L.Q. and S.S. The first draft of the manuscript was written by P.L.Q. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quinn, P.L., Saiyed, S., Hannon, C. et al. Reporting time toxicity in prospective cancer clinical trials: A scoping review. Support Care Cancer 32, 275 (2024). https://doi.org/10.1007/s00520-024-08487-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08487-2