Abstract
Purpose
This study aims to systematically explore the impact of physical exercise as supportive therapy for head and neck cancer.
Methods
A systematic search on PubMed/MEDLINE, Cochrane, and SPORTDiscus was conducted. Randomized controlled trials exploring the effects of a physical exercise intervention in comparison with usual care on outcomes in patients with head and neck cancer were selected. The RoB 2 tool was used to determine the study quality. The extracted data are reported as qualitative synthesis.
Results
Among the 527 records examined, nine studies were included. No trials investigating exercise as prehabilitation were found, whereas eight studies involving 452 patients with head and neck cancer were conducted during anticancer treatment. Most trials did not report improvements in body mass index or body composition, while 2/4 and 3/5 investigations found a significant increase in muscle strength and cardiorespiratory fitness, respectively. Regarding the patients’ reported outcomes, 4 out of 7 studies observed enhancements in some domains of quality of life, and two trials out of 3 detected an amelioration in fatigue following the exercise intervention. Analyzing the exercise programs, it seems that combining aerobic and resistance training could be more beneficial compared to a single type of full-body exercise in counteracting physical decline and controlling symptoms in the anticancer therapy phase. One trial has investigated the effect of resistance exercise on patients who had terminated the anticancer treatments, reporting significant improvements in lean mass, muscle strength, and quality of life.
Conclusion
Exercise may be a promising approach in patients with head and neck cancer. Future studies are needed to consolidate these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to tobacco smoking, alcohol abuse, and oncogenic viruses, including human papillomavirus and Epstein-Barr virus, are recognized risk factors that may lead to developing head and neck cancers [1]. Head and neck malignancies enclose a wide range of cancers that develop in the larynx, pharynx, oral cavity, nasal cavity, and paranasal cavity. With about 931,931 new cases and 467,125 related deaths, head and neck cancer accounts for 4.8% of all tumors and approximately 4.6% of all cancer-related deaths worldwide [2]. To date, treating head and neck cancer requires multidisciplinary expertise, encompassing surgery, radiotherapy, and systemic therapy, in order to offer the best therapeutical approach [1]. Nevertheless, during cancer care, patients with head and neck cancer may experience a series of side effects, such as fatigue, dysphagia, muscle wasting, weight loss, and functional impairments that may seriously affect patients’ quality of life [3, 4]. Additionally, changes in functional parameters and body composition after diagnosis may negatively impact patients’ disease course. For instance, in the surgical context, an observational study including 187 patients undergoing head and neck surgery found that better preoperative cardiorespiratory fitness, an objective measure to assess the individual’s functional capacity, was significantly associated with a decrease in cardiopulmonary complications [5]. Similarly, pre-surgery sarcopenia, i.e., loss of muscle mass, is a strong negative predictor of 2-year and 5-year overall survival in patients with head and neck cancer [6]. Low skeletal muscle mass has been associated with adverse outcomes, also during anticancer treatments. In this sense, sarcopenia represents a negative prognostic factor for overall survival in patients affected by head and neck cancer undergoing radiotherapy or chemoradiotherapy, and it has been significantly correlated with reduced disease-free survival, prolonged radiotherapy breaks, and chemotherapy-related toxicities [7, 8]. Moreover, such muscle impairments may persist for years after therapy conclusion, consequently altering the patients’ strength, functional capacity, and quality of life [9].
In this sense, strategies directed to improve these parameters are fundamental. Nutritional counseling has been suggested as a cornerstone in the management of head and neck cancer. Nevertheless, also exercise may be a useful strategy. Physical exercise has been established as adjunctive therapy across the cancer continuum in several cancer types, able to positively support patients by improving their physical and psychological parameters and accelerating their recovery. Exercise prehabilitation, i.e., the exercise intervention occurring between diagnosis and the start of acute treatments (and often surgery), has been shown to improve strength and cardiorespiratory fitness, reduce postoperative complications, length of hospital stay, and limit the loss of functional impairments in the postoperative phase, in different surgical cancer settings [10, 11]. During anticancer treatments, exercise interventions may improve the health-related components of host physiology, such as cardiorespiratory fitness, strength, and muscle mass [12, 13]. Moreover, exercise has been demonstrated to ameliorate several side effects of anticancer therapies, including fatigue, anemia, lymphoedema, peripheral neuropathy, and sleep quality, and enhance psychological well-being and quality of life [12, 14, 15]. In the survivorship phase, exercise may help recover from residual physical and psychological impairments, as well as preliminary data suggests that being physically active may reduce the recurrence risk [16]. Although several studies and international guidelines support the importance of exercise in cancer, most of the available review and evidence derives from lung, breast, prostate, and colorectal cancer studies. In head and neck cancer, a significant amount of literature and reviews have deeply analyzed the role of specific interventions for trismus, dysphagia, or shoulder dysfunction [17,18,19], whereas little is known about the impact of full-body physical exercise, defined as sessions of muscle strength and/or aerobic exercise. Therefore, this systematic review aims to investigate the impact of full-body physical exercise on physical fitness and patients’ reported outcomes, in patients with head and neck cancer, in the presurgical setting, during anticancer treatments, and after therapy conclusions in order to explore the current data and identify potential knowledge gaps to inform future studies.
Method
Search strategy
On October 13th, 2022, a systematic search was executed through the screening of the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL), PubMed/MEDLINE (National Library of Medicine), and EBSCO Sports Medicine Database (SPORTDiscus). Research headings and keys included those related to head and neck anatomical district (e.g., head and neck, hypopharynx, larynx, oropharynx, oral cavity, or nasopharynx), exercise (e.g., physical activity, physical exercise, exercise prehabilitation, or prehabilitation) and cancer (e.g., tumor or malignancy), were combined using the Boolean operator “AND”. A hand-searched screen was also performed, reviewing the references list of the eligible articles and other narrative and systematic reviews. Further information regarding the literature search is available in the Supplementary Information. The present systematic review was registered on PROSPERO (CRD42022379687) and was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [20].
Study eligibility criteria
Following the PICO tool [21], studies were considered eligible if: i) applied a randomized controlled trial design, ii) included adult patients (age ≥ 18 years) with a confirmed diagnosis of head and neck cancer, iii) investigated a full-body exercise as intervention and utilized as a comparator, a non-exercise intervention (e.g., usual care), or placebo intervention with a minimal likelihood of muscular adaptations (e.g., Qi Qigong, stretching or relaxation), and iv) reported an outcome assessment (physical, psychological, or clinical). Regarding the intervention, exercise, according to Caspersen, was considered a planned, structured, and repetitive body movement to improve or maintain one or more components of physical fitness [22]. Additionally, the exercise intervention had to last at least 3 weeks, i.e., it must be chronic, and each repeated session must have a length of at least 10 min. Exercise interventions were categorized as aerobic, which included any activity, rhythmic in nature, that uses large muscle groups and can be performed continuously, as resistance, i.e., a form of exercise specifically designed to improve muscular strength and mass, or as combined, i.e., including both aerobic plus resistance activities. Exclusion criteria of the studies were: i) non-English full text, ii) applied other forms of physical activity or exercise as a comparator, and iii) interventions targeting specific organ sites or rehabilitation needs (e.g., exercises for tongue, swallow, or shoulder range of motion).
Study selection and data extraction
Studies eligibility was verified through a two-step process. Initially, irrelevant references were removed after screening the title and abstract by two independent authors (A.A. and A.B.), and uncertain studies were carried on for full-text evaluation. The second step consisted of the full-text eligibility evaluation of the selected studies by the same two authors. Any discrepancies during the literature selection were resolved by discussion. For each study, the following relevant data were extracted: i) study details and characteristics: first author name, year of publication, journal of publication, country, study design; primary outcome, and secondary outcomes; ii) population: cancer sites, cancer stage, number of patients affected by human papillomavirus infection, phase of cancer continuum (i.e., prehabilitation, during anticancer treatment or after anticancer treatments), percentage of patients undergoing active anticancer treatment, number of patients allocated in the treatment group, number of patients allocated as controls; iii) exercise intervention characteristics: length of the intervention, supervision of the intervention, type of intervention, frequency, duration, intensity, progression, presence of nutritional support/intervention, presence of other interventions, type of controls; and iv) outcomes: recruitment rate, adherence rate (i.e., the percentage of training sessions successfully completed to the planned), dropouts (i.e., the ratio of patients did not complete post-intervention assessments), safety profile (i.e., the occurred adverse events of any grade), summary of the study results. To extract and collect data, a Microsoft Excel spreadsheet summary was created.
Risk of bias assessment
The methodological quality of each included study was determined, by two independent authors (A.A. and A.B), utilizing the Cochrane Collaboration’s Risk of Bias tool 2nd version (RoB 2) [23]. The Rob2, specifically used for randomized controlled trials, assesses the following domains: i) bias arising from the randomization process; ii) bias due to deviations from intended interventions; iii) bias due to missing outcome data; iv) bias in the measurement of the outcome; and v) bias in the selection of the reported result. The summary of the risk of bias is categorized as "low risk of bias", "some concerns", or "high risk of bias" [23].
Data synthesis
Given the scarcity of the amount of the literature and the considerable heterogeneity across studies in outcomes definition and exercise prescription, a meta-analysis was not conducted. A qualitative synthesis, in narrative form, is provided, focusing on the different timing of the cancer continuum, i.e., prior surgery (e.g., prehabilitation), during anticancer treatments, and after anticancer treatments. The extracted results were examined using tables and a narrative description, grouping data according to characteristics, as suggested by the Guidance on the Conduct of Narrative Synthesis in Systematic Reviews [24, 25].
Results
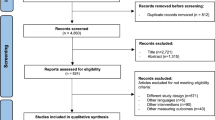
Among the 527 records examined for title and abstract, 506 were excluded (Fig. 1). A total of 21 research articles were screened for full-text evaluation, and among these, nine met the inclusion criteria. The reasons for the full-text exclusion are presented in the Supplementary Information.
Characteristics of the included studies and quality assessment
The general characteristics of the included studies are presented in Table 1. Briefly, two trials were conducted in the US [26, 27], two in India [28, 29], two in Taiwan [30, 31], and one each in Canada [32], Germany [33] and Denmark [34]. Overall, seven trials applied a randomized controlled trial design [26,27,28,29,30,31] [33], whereas two performed a cross-over randomized controlled trial [32] [34]. The sample size across the studies varied from 15 to 148 patients. Six research articles specified the cancer sites across the head and neck district [26, 27, 29, 31, 32, 34], and the disease stage varied from stage I to IV, with two trials that did not report it [28, 30]. Overall, the risk of bias assessment revealed some concerns for eight trials, whereas, in one investigation, the risk of bias was high (Supplementary Information).
Exercise before surgery
No randomized controlled trials have currently explored the effect of exercise as prehabilitation in patients with head and neck cancer.
Exercise during oncological treatments
Eight trials have investigated the effect of exercise in patients with head and neck cancer during active anticancer treatments [26,27,28,29,30,31,32,33]. A total of three studies were conducted on patients treated with radiation or chemoradiotherapy [26, 32, 33], three in patients scheduled for chemoradiotherapy [27,28,29], and two in those undergoing chemotherapy [30, 31].
Regardless of exercise prescription (Table 2.), the length of intervention ranged from 7 to 12 weeks. Five out of eight studies proposed a combined exercise, i.e., aerobic plus resistance training [27,28,29,30,31], whereas three focused on resistance training only [26, 32, 33]. The exercise frequency varied 2–5 days a week, and each session lasted approximately 30–90 min. Two studies did not report the exercise intensity [26, 32, 33], whereas four investigations declared to have performed a progressive exercise prescription [26, 31,32,33]. Concerning supervision, three studies stated to start the exercise intervention with a supervised phase followed by an unsupervised period [26, 27, 29], three proposed a supervised program [28, 31, 33], and one a mixed program with supervised and unsupervised sessions in the same week [32].
Safety and feasibility
Feasibility was the primary aim in three trials [26, 27, 33], assessed through recruitment rate, adherence rate to the exercise intervention, and withdrawals. Six out of eight trials reported a recruitment rate ranging from 20 to 32.3% [26, 28, 29, 32, 33, 35]. Adherence to the exercise intervention was provided by five studies and varied from 45.2% to 93.1% [26, 27, 31,32,33]. Only one investigation did not report information regarding withdrawal [33]. Dropout rates among the patients allocated in the intervention group ranged from 0 to 38.7%, whereas for controls varied between 0 and 28.6% [26,27,28,29,30,31,32]. Regarding safety, only three studies reported the safety profile of exercise [26, 28, 33]. Samuel and colleagues stated that no adverse events occurred during the study [28], whereas in the investigations of Grote et al. [33] and Rogers et al. [26], non-exercise-related adverse events were reported (Table 2).
Anthropometric measures and body composition
Five studies have analyzed the effect of an exercise intervention on body mass index (BMI) (Table 3.). One trial reported a significant decline in BMI in the intervention groups compared to the controls [30], while one investigation found a maintenance of the BMI [31]. Three studies reported a decline in BMI in the intervention groups, which was not significantly different from this experienced by the controls [26, 27, 32].
Body composition components were evaluated in six trials using dual-energy x-ray absorptiometry [27, 32] or bioimpedance analysis [26, 30, 31, 33]. Focusing on lean body mass, four investigations did not observe significant differences, whereas the remaining two found significant changes. Among those that did not report significative improvements, two studies exploring the effect of 12 weeks of progressive resistance training versus usual care reported a non-statistically significative decline in lean body mass in both intervention and control groups [26, 32], one detected an increase in the intervention and a slight decrease in the controls, but the differences did not result to be significative [33]; another one, which tested the combination of 14-week of supervised and unsupervised walking and resistance exercises found non-significant improvements in both groups [27]. On the other hand, two studies that proposed a combined aerobic and resistance exercise training observed significant improvement in skeletal muscle mass [30, 31]. For instance, Lin and colleagues proposed moderate-intensity aerobic training on the treadmill and resistance exercises using elastic bands or free weights to perform three times per week. After eight weeks, although the changes within groups were not significant (interventional group (IG): 34.1 ± 3.4% pre-intervention, 34.5 ± 2.4% post-intervention, p = 0.61 vs. control groups (CG): 31.5 ± 2.9% pre-intervention, 31.4 ± 2.4% post-intervention, p = 0.87), the difference between groups has reached the statistical significance (p = 0.008) [31].
The fat component was reported in four trials, two observed a non-significant decline in both groups [32, 33]. Instead, a study including 84 patients found that 8 weeks of aerobic and resistance training at moderate intensity was able to reduce visceral fat (IG:7.9 ± 4.7 pre-intervention, 7.4 ± 4.5 post-intervention p < 0,05 vs. CG: 8.2 ± 5.5 pre-intervention, 7.8 ± 5.7 post-intervention, p-value not reported) but did not produce significative alterations in the total body fat [30]. On the contrary, the just mentioned trial by Lin et al., which proposed a similar exercise intervention in 57 patients, found a decrease in the total body fat, which resulted in a significant difference between groups (IG: 25.5 ± 4.0 pre-intervention, 21.0 ± 2.8 post-intervention vs. CG: 25.9 ± 3.5 pre- intervention, 25.8 ± 2.5 post-intervention, p = 0.002), but did not observe alterations in visceral body fat [31].
Physical function measures
Different outcomes related to physical function were evaluated in response to exercise intervention, including cardiorespiratory fitness, muscle strength, flexibility, and balance (Table 3.).
Cardiorespiratory fitness was indirectly estimated using the “six minutes walking test” in five trials [27,28,29,30, 32] and the “three minutes step test" in one investigation [31]. A 6-week supervised resistance training followed by 6 other weeks of home-based resistance exercises did not produce improvements in cardiorespiratory fitness, but even a decline was reported in both groups, interventional and control (mean change from baseline to post-intervention: IG: − 13 ± 19.6 m vs. − 35.4 ± 18.7 m (p = 0.59) [33]. Two investigations reported an increase in cardiorespiratory fitness among the interventional groups and a decrease in the controls, but the differences were not statistically significant [27, 31]. Three trials found that a combined aerobic and resistance exercise intervention significantly improved cardiorespiratory fitness compared to usual care [28,29,30]. For instance, an 11-week (7 weeks of supervised followed by 4 weeks of home-based intervention) of 15–20 min of aerobic plus resistance training performed at a moderate intensity 5 days per week increased by 37 m the performance in the “Six minutes walking test”, whereas the controls experienced a decrease of 73 m, resulting in a significant difference between groups (IG: 446.31 ± 62.87 m pre-intervention, 483.16 ± 88.24 m post-intervention vs. CG: 447.32 ± 59.22 m pre-intervention, 374.52 ± 110.26 m post-intervention, p < 0.001) [29].
Muscular strength was assessed in four trials [26, 27, 31,32,33], using isokinetic dynamometers, functional tests, such as “30 s arm curl and chair stand”, and the handgrip strength test. Two studies that tested 12 weeks of resistance training did not find improvements in muscle strength [26, 32]. In the study of Capozzi et al., a 12-week resistance training performed four times per week was unable to preserve muscular strength since, in the post-intervention evaluations, a decline in both groups, intervention, and control, was observed [33]. Similarly, in the investigation of Rogers and colleagues, even if a reduction was not detected, no significant changes were reported in the strength measures [26]. Instead, two trials combining aerobic training with resistance exercises found improvements in muscle strength measures [27, 31]. In the study of Lin et al., after exercise intervention, a significant difference between groups in favor of exercise groups was observed for the upper limb (IG: 24.1 ± 6.3 repetitions pre-intervention, 27.0 ± 5.8 repetitions post-intervention vs. CG: 23.4 ± 8.4 repetitions pre-intervention, 21.06 ± 5.38 repetitions post-intervention, p = 0.037) and lower limb strength (IG: 19.7 ± 6.58 repetitions pre-intervention, 20.14 ± 7.04 repetitions post-intervention vs. CG: 15.6 ± 4.37 repetitions pre-intervention, 13.1 ± 3.87 repetitions post-intervention, p = 0.025) [31]. Zhao and colleagues, on the one side, did not detect differences in upper limb strength but reported a maintenance in knee extensor strength for the exercise groups and a decline in the controls, finally resulting in a significant difference between groups (mean change from baseline to post-intervention, IG: − 4 ± 7 N-m vs. CG: − 46 ± 14 N-m, p < 0.05) [27].
Balance and flexibility were assessed in two studies each. Regarding balance, any intervention has produced improvements [27, 31], whereas for flexibility, one investigation reported no significant change [32], and one found an increase in upper limb flexibility [31].
Other outcomes
Across the studies, blood pressure, resting heart rate, the total count of hemoglobin and platelets, and treatment toxicities were evaluated once (Table 3.). In the study of Zhao et al. [27], treatment toxicities, assessed according to the Common Toxicity Criteria for Adverse Events, did not differ between exercise and control groups. Similarly, the levels of hemoglobin and platelets did not result in a significant change between the two groups, which exhibited a decline over time [29]. In the study of Yen and colleagues, the 8-week exercise intervention produced a significant reduction in rest systolic (IG: 114.4 ± 18.7 mmHg pre-intervention, 104.2 ± 11.1 mmHg post-intervention vs. CG: 120.7 ± 18.3 mmHg pre-intervention, 116.0 ± 15.6 mmHg post-intervention, p < 0.05) and diastolic pressure (IG: 70.9 ± 13.3 mmHg pre-intervention, 61.9 ± 8.9 mmHg post-intervention vs. CG: 74.3 ± 12.7 mmHg pre-intervention, 75.8 ± 10.3 mmHg post-intervention, p < 0.05), as well as in resting heart rate (IG: 83.3 ± 12.4 bpm pre-intervention, 73.5 ± 11.1 bpm post-intervention vs. CG: 77.9 ± 16.5 bpm pre-intervention, 83.2 ± 17.8 bpm post-intervention, p < 0.05) [30].
Patient-reported outcomes
The quality of life was examined in seven out of eight trials (Table 3.). Different scales were used, including Functional Assessment of Cancer Therapy (FACT)-Anemia [32], Functional Assessment of Cancer Therapy Head and Neck Cancer Symptom Index (FHNSI)—22 [32], Short Form Health Survey 36 (SF-36) [27,28,29], Functional Assessment of Anorexia/cachexia Therapy (FAACT) [33], European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 [31], FACT—Head and Neck [26], FACT—general [26], EORTC QLQ Head and Neck 35 [31]. Two studies that proposed resistance training as an intervention did not report enhancements in quality of life domains [32, 33]. Three investigations exploring combined aerobic and resistance training reported an improvement in the mental health variable measured with the SF-36 [27,28,29], while one of these also found an increase in the physical component [29]. Rogers described an increase in emotional well-being and no significant change in fatigue, physical functioning, social well-being, functional well-being, and the total score [26]. In the study of Lin and colleagues, the exercise group exhibited a better profile in the global health status, physical, role, and emotional functioning, appetite loss, and fatigue compared to controls [31]. Additionally, two studies assessed fatigue using specific tools. Grote and colleagues did not find improvement in the fatigue variables using the Multidimensional Fatigue Inventory [33], whereas a significant decrease was reported in the study of Samuel et al., which evaluated it with the National Comprehensive Cancer Network numeric rating scale 0–10 (IG: 3.70 ± 1.75 point pre-intervention, 2.45 ± 1.97 point post-intervention vs. CG: 2.91 ± 1.84 point pre-intervention, 4.48 ± 1.59 point post-intervention, p < 0.001) [31]. Depression, nutritional status, and sleep were assessed in one study each; no significant improvements were detected [27, 32].
Exercise after oncological treatments
One trial included patients affected by head and neck cancer after the oncologic treatment phase [34] (Table 3.). In this cross-over randomized controlled trial, patients were randomized to participate in an early exercise intervention (EAI) composed of 12 weeks of progressive resistance training or usual care (self-chosen physical activity). Subsequently, the groups reversed the interventions, i.e., patients enrolled in the self-chosen physical activity underwent resistance training (delayed exercise intervention—DEI), and the EAI group acted as a control. The study reported a recruitment rate of 22%, an overall adherence to resistance training of 95%, and no adverse events were registered. After the first 12 weeks, the EAI group increased by 4.3% lean body mass, while the DEI patients had a change of 1.3% (p = 0.0005). After the last 12 weeks, the DEI groups experienced a gain of 4.2% in lean body mass, while in the EAI only a 0.5% improvement was detected (p < 0.0001). Similar results were obtained for muscle strength, evaluated with isometric and isokinetic knee extensors and flexors, and aerobic capacity measured with the stair climb test, which significantly increased in EAI compared to DEI after the first 12 weeks, while after the last 12 weeks showed a reverse trend, i.e., significantly improve in the DEI than EAI group. Regarding the quality of life, resistance training intervention enhanced the global health status and cognitive function in the EAI, and improved physical function in the DEI group. Considering the entire study period, i.e., 24 weeks, both interventions exhibited improvements in lean body mass, strength, aerobic capacity, and quality of life without differences between groups [34].
Discussion
This systematic review provides a comprehensive overview of trials investigating the role of full-body physical exercise after a diagnosis of head and neck cancer. A total of nine randomized controlled trials were identified, conducted during oncological treatment (n = 8), after the conclusion of anticancer therapies (n = 1), whereas none was performed before treatments, i.e., as prehabilitation.
The last update of the Enhanced Recovery After Surgery (ERAS) guidelines highlighted the overall aim to improve early functional recovery in patients undergoing major head and neck cancer surgery [36]. Among the proposed multimodal interventions, prehabilitation, aiming to boost the patient’s condition before surgery, plays a key role. In the last ERAS guidelines for head and neck cancer surgery, prehabilitation has primarily focused on optimizing nutritional status and intervention to reverse malnutrition or diminish its risk, whereas no mention was made of exercise [36]. It is not surprising, given the absence of evidence in this setting. However, preoperative exercise might be an important intervention to increase muscle mass and cardiorespiratory fitness, which are prognostic factors before head and neck cancer surgery [5, 6]. Moreover, from experiences in other surgical contexts, including thoracic or abdominal surgery, preoperative exercise may reduce postoperative complications and length of hospital stay and diminish the loss of function [10, 11]. It is possible to speculate that these benefits may also be achieved in people affected by head and neck cancers. Three clinical trials exploring the impact of exercise as prehabilitation are currently ongoing to address these issues (Table 4.). These investigations will probably pave the way for exercise prehabilitation in the head and neck cancer setting, provide important preliminary information, and extend the base of evidence about the potential contribution of exercise in this setting.
Regarding exercise intervention during and after anticancer treatments, the adherence rate across the studies was highly heterogeneous, suggesting that future studies are needed to explore programs addressed to increase this outcome. The optimization of adherence to an exercise program is crucial, particularly because a head and neck cancer diagnosis profoundly impacts patients, also affecting their lifestyle. Indeed a survey by Rogers and colleagues reported that if 30.5% of patients met the exercise guidelines before the disease, such percentage drastically dropped to 8.5% after diagnosis [37]. Despite the high prevalence of insufficient exercise levels, patients with head and neck cancer are interested in exercising, with 75% willing to start an exercise program [38]. Barriers related to exercise may influence the adoption and maintenance of an active lifestyle. Patients may face a series of obstacles, such as dry mouth, difficulty eating, pain, fatigue, and shortness of breath, which are typically the direct consequences of head and neck anticancer treatments [39]. In this sense, adapting the exercise program considering these issues and opting for a multidisciplinary management, e.g., simultaneous care and nutritional counseling, may be fundamental. Moreover, the fear of injury has emerged as another non-treatment-related factor potentially hindering adherence to an exercise program [39]. In this review, only three trials have investigated the safety of the exercise intervention. Although no adverse events directly attributable to exercise have been reported in these three studies, it is hard to state the safety of exercise, since the small sample size of the trials. Generally, exercise-related adverse events are rare but can be significant if they occur. In this sense, large studies with detailed adverse events reporting are needed to conclude safety with any certainty.
Studies investigating exercise during oncological treatments reported mixed results on head and neck cancer outcomes. Focusing on the type of exercise training, it seems that resistance training alone can not produce significant improvements in lean body mass, cardiorespiratory fitness, strength, fatigue, and quality of life [32, 33, 37]. Instead, combined aerobic and resistance training appears more beneficial and capable of increasing the outcomes mentioned above [28,29,30,31]. Whereas for the enhancements of some outcomes, such as cardiorespiratory fitness, aerobic training is fundamental, for others, the superiority of the combined aerobic and strength intervention with respect to the resistance training alone may seem unusual. This is the case, for instance, of muscle mass. Indeed, the gain of muscle mass is highly stimulated by resistance training but surprisingly, the studies that investigated resistance training as an intervention did not find improvements, while the combination of aerobic and resistance has been shown to promote protein synthesis. A possible explanation could be related to the control of inflammatory levels. Inflammation is associated with weight loss in patients with head and neck cancer [40], and it is a recognized hallmark of cancer cachexia [41]. Aerobic exercise may have an anti-inflammatory role, able to counteract catabolism and synergistically enhance the anabolic effect of strength training [42]. Since patients affected by head and neck cancer are at high risk of developing cachexia and frequently experience a severe depletion in skeletal muscle mass during treatments, impacting survival, quality of life, and treatment tolerance [43], finding approaches, such as full body physical exercise, to offset the usual deterioration is fundamental.
Finally, regarding the post-treatment phase, just one study has been published [34]. In this context, progressive resistance training improved lean body mass, strength, and quality of life. Nevertheless, it is necessary to increase the number of investigations as well as the proposed interventions and the outcomes evaluated to understand the real potential of exercise during the recovery phase of head and neck cancer.
The present review has limitations that should be noted. Only a limited number of studies with small sample sizes, to date, have explored the effect of exercise in patients with head and neck cancer during anticancer treatments. This may suggest to cautiously interpret the results since most studies were not appropriately powered to detect possible clinically relevant differences in all outcomes. Investigations testing the effect of full-body physical exercise as prehabilitation or during survivorship are practically absent. Moreover, heterogeneity in included disease subtypes and assessment tools emerged across the investigations, making the results difficult to compare and generalize.
Conclusion
In summary, full-body physical exercise, mainly if composed of aerobic and resistance components, may help to mitigate some side effects commonly experienced by patients with head and neck cancers during anticancer therapies. Moreover, the present systematic review highlights the necessity to implement future studies with a solid design to investigate those underexplored areas, such as the prehabilitation one, and fully understand the role of exercise in supporting patients with head and neck cancer.
Data availability
The data are available upon request from the authors.
Code availability
N/A.
References
Mody MD et al (2021) Head and neck cancer. Lancet 398(10318):2289–2299
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Silver HJ, Dietrich MS, Murphy BA (2007) Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 29(10):893–900
do Nascimento Santos Lima E et al (2020) Health-related quality of life became worse in short-term during treatment in head and neck cancer patients: a prospective study. Health Qual Life Outcomes 18(1):307
Lalabekyan BB et al (2021) Cardiopulmonary exercise testing and cardiopulmonary morbidity in patients undergoing major head and neck surgery. Br J Oral Maxillofac Surg 59(3):297–302
Stone L et al (2019) Association between sarcopenia and mortality in patients undergoing surgical excision of head and neck cancer. JAMA Otolaryngol Head Neck Surg 145(7):647–654
Findlay M et al (2020) The association between computed tomography-defined sarcopenia and outcomes in adult patients undergoing radiotherapy of curative intent for head and neck cancer: a systematic review. J Acad Nutr Diet 120(8):1330-1347.e8
Baxi SS, Schwitzer E, Jones LW (2016) A review of weight loss and sarcopenia in patients with head and neck cancer treated with chemoradiation. Cancers Head Neck 1:9
Oates JE et al (2007) Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 133(6):533–540
Avancini A et al (2021) Exercise prehabilitation in lung cancer: getting stronger to recover faster. Eur J Surg Oncol 47(8):1847–1855
Thomas G et al (2019) Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol 36(12):933–945
Campbell KL et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390
Avancini A et al (2022) Effect of exercise on functional capacity in patients with advanced cancer: a meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol 175:103726
Ligibel JA et al (2022) Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol 40(22):2491–2507
Avancini A et al (2021) Exercise and anemia in cancer patients: could it make the difference? Expert Rev Hematol 14(11):979–985
Cormie P et al (2017) The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev 39(1):71–92
Shao CH, Chiang CC, Huang TW (2020) Exercise therapy for cancer treatment-induced trismus in patients with head and neck cancer: a systematic review and meta-analysis of randomized controlled trials. Radiother Oncol 151:249–255
Yang W et al (2021) Review of prophylactic swallowing interventions for head and neck cancer. Int J Nurs Stud 123:104074
Almeida KAM et al (2020) Rehabilitation interventions for shoulder dysfunction in patients with head and neck cancer: systematic review and meta-analysis. Phys ther 100(11):1997–2008
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med 6(7):e1000097
Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100(2):126–131
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Popay J et al (2006) Guidance on the conduct of narrative synthesis in systematic Reviews. A product from the ESRC methods programme. Version 1
Dillekas H, Rogers MS, Straume O (2019) Are 90% of deaths from cancer caused by metastases? Cancer Med 8(12):5574–5576
Rogers LQ et al (2013) Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 35(8):1178–1188
Zhao SG et al (2016) Maintaining physical activity during head and neck cancer treatment: results of a pilot controlled trial. Head Neck 38(Suppl 1):E1086–E1096
Samuel SR et al (2013) Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res 137(3):515–520
Samuel SR et al (2019) Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support Care Cancer 27(10):3913–3920
Yen CJ et al (2019) Multimodal exercise ameliorates exercise responses and body composition in head and neck cancer patients receiving chemotherapy. Support Care Cancer 27(12):4687–4695
Lin KY et al (2021) Effects of exercise in patients undergoing chemotherapy for head and neck cancer: a pilot randomized controlled trial. Int J Environ Res Public Health 18(3)
Capozzi LC et al (2016) Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: results from an exploratory randomized controlled exercise trial. Cancer 122(8):1185–1200
Grote M et al (2018) Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial. Radiat Oncol 13(1):215
Lønbro S et al (2013) Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy–results from the randomized DAHANCA 25B trial. Radiother Oncol 108(2):314–319
Antrim L et al (2021) Impact of COVID-19 infection among cancer patients treated at the Los Angeles County Medical Center. Cancer Treat Res Commun 26:100273
Dort JC et al (2017) Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the Enhanced Recovery After Surgery Society. JAMA Otolaryngol Head Neck Surg 143(3):292–303
Rogers LQ et al (2006) Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer 14(10):1012–1019
Rogers LQ et al (2009) Exercise preferences among patients with head and neck cancer: prevalence and associations with quality of life, symptom severity, depression, and rural residence. Head Neck 31(8):994–1005
Rogers LQ et al (2008) Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer 16(1):19–27
Kubrak C et al (2013) Clinical determinants of weight loss in patients receiving radiation and chemoirradiation for head and neck cancer: a prospective longitudinal view. Head Neck 35(5):695–703
Avancini A et al (2021) A multimodal approach to cancer-related cachexia: from theory to practice. Expert Rev Anticancer Ther 21(8):819–826
Lira FS, Neto JC, Seelaender M (2014) Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab 39(6):679–686
Ferrão B et al (2020) Body composition changes in patients with head and neck cancer under active treatment: a scoping review. Support Care Cancer 28(10):4613–4625
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Alice Avancini, Sara Pilotto, and Anita Borsati. Analysis was performed by Alice Avancini. The first draft of the manuscript was written by Alice Avancini and all authors commented on the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A.
Consent for publication
N/A.
Competing interests
SP reports personal fees from AstraZeneca, Eli-Lilly, Novartis, AMGEN, BMS, Boehringer Ingelheim, Merck & Co. and Roche; and grants from AstraZeneca and BMS outside the submitted work. MM reports personal fees from Pfizer, MSD, AstraZeneca, EUSA Pharma, Boehringer Ingelheim and Ipsen; and grants from Roche and BMS, outside the sub mitted work. All remaining authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avancini, A., Borsati, A., Belluomini, L. et al. Effect of exercise across the head and neck cancer continuum: a systematic review of randomized controlled trials. Support Care Cancer 31, 670 (2023). https://doi.org/10.1007/s00520-023-08126-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08126-2