Abstract
Purpose
The aim of this study was to evaluate quality of life (QoL) in patients with gastric adenocarcinoma receiving adjuvant chemoradiotherapy (CRT).
Methods
The European Organization for Cancer Research and Treatment Quality of Life Questionnaire-Core 30 (QLQ-C30) and site-specific module for gastric cancer (QLQ-STO22) were administered at four time points to 156 patients admitted to Cumhuriyet University Oncology Center between 2011 and 2018.
Results
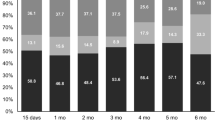
The patient group comprised 76% men and 24% women with a median age of 61 years (range, 18–88). During CRT, 12 patients (8%) discontinued treatment, 25 (16%) lost weight, and 42 (27%) had reduced performance. There was significant worsening in QLQ-C30 global health status and all functional and symptom scale scores at CRT completion. These changes were also clinically significant except for physical functioning scores and were supported by minimal clinically important difference measurements. In the QLQ-STO22, all symptoms except dry mouth and hair loss were negatively affected at CRT completion. In general, scores were improved at 1 month after CRT and almost all scores reached baseline level by 6 months. Certain scores were more adversely affected in women (global health status, physical functioning, role functioning, fatigue, pain, and insomnia), those who lost weight during CRT (emotional functioning), and those with CRT interruption (emotional functioning and anxiety).
Conclusion
Although CRT reduces QoL in patients with gastric cancer, the effects tend to resolve within 6 months after completing treatment. Female sex, weight loss, and CRT interruption negatively affected some QoL scores.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bollschweiler E, Berlth F, Baltin C, Mönig S, Hölscher AH (2014) Treatment of early gastric cancer in the Western world. World J Gastroenterol 20(19):5672–5678. https://doi.org/10.3748/wjg.v20.i19.5672
Gunderson LL, Sosin H (1982) Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 8:1. https://doi.org/10.1016/0360-3016(82)90377-7
Park SH, Kim DY, Heo JS, Lim DH, Park CK, Lee KW et al (2003) Postoperative chemoradiotherapy for gastric cancer. Ann Oncol 14(9):1373–1377. https://doi.org/10.1093/annonc/mdg366
Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM et al (2015) Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 33(28):3130–3136. https://doi.org/10.1200/JCO.2014.58.3930
Kassam Z, Mackay H, Buckley CA, Fung S, Pintile M, Kim J, Ringash J (2010) Evaluating the impact on quality of life of chemoradiation in gastric cancer. Curr Oncol 17(4):77–84. https://doi.org/10.3747/co.v17i4.522
Pruthi DS, Ahmad M, Gupta M, Bansal S, Nautiyal V, Saini S (2018) Assessment of quality of life in resectable gastric cancer patients undergoing chemoradiotherapy as adjuvant treatment. South Asian J Cancer 7(1):16–20. https://doi.org/10.4103/sajc.sajc_196_17
Aaronson NK, Cull AM, Kaasa S, Sprangers MAG (1996) The European Organization of Research and Treatment of Cancer (EORTC) modular approach to quality of life assessment in oncology: and update. In: Spilker B (ed) Quality of Life and Pharmacoeconomics in Clinical Trials, 2nd edn. Raven Press, New York, NY, pp 179–190
Gotay CC, Wilson M (1998) Use of quality-of-life outcome assessments in current cancer clinical trials. Eval Health Prof 21:157–178. https://doi.org/10.1177/016327879802100203
Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, Kvikstad A (1995) The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer 31A:2260–2263. https://doi.org/10.1016/0959-8049(95)00296-0
Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S (1995) Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol 13:1249–1254. https://doi.org/10.1200/JCO.1995.13.5.1249
Cankurtaran ES, Ozalp E, Soygur H, Ozer S, Akbiyik DI, Bottomley A (2008) Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. Eur J Cancer Care 17(1):98–104. https://doi.org/10.1111/j.1365-2354.2007.00827.x
Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O et al (2004) Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer 40:2260–2268. https://doi.org/10.1016/j.ejca.2004.05.023
Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, Bae JM, Yun YH, Kim S (2012) Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg 256(6):1008–1013. https://doi.org/10.1097/SLA.0b013e31827661c9
Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A (2011) Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg 35(2):357–364. https://doi.org/10.1007/s00268-010-0860-2
Tyrvainen T, Sand J, Sintonen H, Nordback I (2008) Quality of life in the long-term survivors after total gastrectomy for gastric carcinoma. J Surg Oncol 97:121–124. https://doi.org/10.1002/jso.20925
Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, Wang-Peng J (2008) Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer 98:54–59. https://doi.org/10.1038/sj.bjc.6604097
Li J, Liu H, Yang G, Chen S (2018) Quality of life after esophagogastrostomy plus gastrojejunostomy reconstruction following proximal gastrectomy: a comparative study of three surgical procedures. Int J Clin Exp Med 11 (9):9791–9801. www.ijcem.com/ ISSN:1940-5901/IJCEM0056192
Lee MK, Lee KM, Bae JM, Kim S, Kim YW, Ryu KW et al (2008) Employment status and workrelated difficulties in stomach cancer survivors compared with the general population. Br J Cancer 98:708–715. https://doi.org/10.1038/sj.bjc.6604236
Bae JM, Kim S, Kim YW, Ryu KW, Lee JH, Noh JH et al (2006) Health-related quality of life among disease-free stomach cancer survivors in Korea. Qual Life Res 15:1587–1596. https://doi.org/10.1007/s11136-006-9000-8
Amin MB, Edge SB, Greene FL (2017) AJCC cancer staging manual, 8th edn. Springer, New York, NY
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z (2003) Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22(3):321–336. https://doi.org/10.1016/s0261-5614(02)00214-5
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346. https://doi.org/10.1016/0360-3016(95)00060-C
Fayers P, Aaronson NK, Bjordal K, Sullivan M (1995) EORTC QLO-C30 scoring manual. EORTC Data Center, Belgium 8.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376. https://doi.org/10.1093/jnci/85.5.365
Wright A, Hannon J, Hegedus EJ, Kavchak AE (2012) Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther 20(3):160–166. https://doi.org/10.1179/2042618612Y.0000000001
Şeker A (2020) Irrationality of gender inequality and reflections in the public sphere (Toplumsal cinsiyet eşitsizliğinin irrasyonelliği ve kamusal alandaki yansımaları). Curr Res Soc Sci 6(2):92–102. https://doi.org/10.30613/curesosc.651457
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mukaddes Yılmaz. The first draft of the manuscript was written by Mukaddes Yılmaz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Sivas Cumhuriyet (Date 19.08.2021/No. 2021–08/47).
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yılmaz, M., Erdiş, E., Uçar, M. et al. “Evaluation of quality of life in patients with gastric adenocarcinoma receiving chemoradiotherapy: a cross-sectional study”. Support Care Cancer 31, 600 (2023). https://doi.org/10.1007/s00520-023-08036-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08036-3