Abstract
Purpose
Although mobile-based symptom monitoring is expected to improve patient participation in symptom management during anticancer therapy, previous trials have not evaluated its effectiveness. Therefore, this study aims to evaluate the impact of a symptom monitoring mobile application on improving patient participation in symptom management during anticancer therapy.
Methods
We conducted a single-center, open-label, randomized controlled trial that enrolled patients with breast, lung, head and neck, esophageal, or gynecologic cancer who were scheduled to receive anticancer therapy (oral or intravenous) between October 2020 and March 2021. We excluded patients with physical or psychological problems. The intervention group received a symptom monitoring application for 8 weeks, and the control group received the usual clinical practice. At 8 weeks, the improvement in patient participation in symptom management was assessed, and additionally quality of life and unplanned clinical visits were assessed.
Results
A total of 222 patients were included in the analysis, of whom 142 were randomly assigned to the intervention group and 71 to the control group. The intervention group reported better outcome in patient participation in symptom management than the control group at 8 weeks (mean scores of 8.5 vs. 8.0; P = 0.01). There were no significant differences between the groups in Quality of life (P = 0.88) and unplanned clinical visits (P = 0.39–0.76).
Conclusions
This study is meaningful in figuring out that the mobile-based symptom monitoring made them more engaged in their management. Future research should continue to evaluate the effects of patient participation as mediators of clinical outcomes.
Trial registration
ClinicalTrials.gov NCT04568278.
Similar content being viewed by others
Introduction
Nearly 90% of cancer patients report at least one physical or psychological problem during anticancer therapy [1,2,3]. These symptoms can significantly impact a patient’s health-related quality of life (HRQoL) and may negatively affect the planned treatment [4,5,6,7]. To ensure effective symptom monitoring and management, the use of patient-reported outcome (PRO) measures is recommended in the routine clinical practice [8,9,10,11]. However, effects of PRO on symptom monitoring have been obtained under conditions with the 24/7 availability of a clinical trial nurse. This may lead to a vague understanding of the importance of individual capability or willingness in managing symptoms.
With the COVID-19 pandemic increasing interest in remote monitoring systems in the healthcare sector [12], several PRO symptom monitoring systems using digital technologies have been developed [13,14,15]. In particular, clear evidence has been reported regarding the benefits of electronic PRO symptom monitoring (ePRO) in cancer patients. ePRO showed a positive effect on the HRQoL of patients and patient satisfaction with care [13,14,15,16,17]. It also led to a substantial decrease in healthcare usage, preventing unplanned emergency room visits and hospital readmissions [13]. Furthermore, evidence illustrates that ePRO has an outstanding survival benefit for patients with advanced cancer [18, 19].
A key benefit of the implementation of ePRO symptom monitoring is expected to be an improvement in patient participation in symptom management [20]. Patient-centered e-health services are generally expected to facilitate self-management and empowerment [21]. E-health functions as a lever for engaging health professionals in working together with patients, thus promoting better self-management and individual responsibility on the part of the patient [21, 22]. For this reason, ePRO is expected to improve patient participation [23]. However, previous randomized controlled trials on ePRO have not evaluated the improvement of patient participation, which is an important mediator in regular clinic visits [4, 13, 15, 18, 19, 24].
We conducted a randomized controlled trial to evaluate the effect of a mobile-based symptom monitoring tool on patient participation in symptom management during anticancer therapy, as well as its impact on health-related quality of life (HRQoL) and the incidence of emergency room visits, unplanned outpatient visits, and unplanned hospitalizations. For this trial, we used the electronic Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) mobile application (ePRO-CTCAE app). Our hypotheses were that cancer patients who were provided with the ePRO-CTCAE app during anticancer therapy would be more willing to participate in self-symptom management compared to those who were not provided with the app. Additionally, we hypothesized that the intervention group would have greater HRQoL and experience fewer emergency room visits, unplanned outpatient visits, and unplanned hospitalizations due to symptoms or treatment toxicities during the study period.
Methods
Trial design and participants
This study was a single-center, open-label, randomized controlled trial (ClinicalTrials.gov NCT04568278) conducted from October 2020 to March 2021 at the Samsung Medical Center in Seoul, Korea.
We recruited patients over 18 years of age who were diagnosed with breast, lung, head and neck, esophageal, or gynecologic cancer and scheduled to receive chemotherapy and/or radiation therapy and who had their smartphone (Android operating system only). Patients scheduled to receive chemotherapy were recruited regardless of the administration of the treatment (oral or intravenous) and treatment intent (curative or palliative). Patients with a history of severe cognitive impairment (e.g., dementia), psychological problems (e.g., depression), or physical problems (e.g., blindness) to use ePRO-CTCAE app were excluded based on the electronic medical records (EMRs). Those with a life expectancy of fewer than 6 months were also excluded. The study was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020–04-215–004), and all study participants provided written informed consent. The full protocol for the study was submitted in the IRB. In addition, the protocol was registered in ClinicalTrials.gov (register number: NCT04568278) prior to enroll a patient.
Randomization and blinding
A random allocation sequence was generated using Sealed Envelope Ltd. 2021 by a statistician who was not involved in the recruitment procedure. Since remote symptom monitoring has been demonstrated to improve outcomes in previous studies, consenting patients were randomly assigned 2:1 to the intervention or usual clinical practice, stratified by cancer site, in random permuted blocks (variable block sizes 3, 6) [25]. The sequentially numbered participants were concealed from the research personnel, who enrolled and assigned the participating patients to their groups. Neither patients nor researchers could be blinded because of the nature of the intervention. However, the statisticians who developed the random blocks and conducted the statistical analyses were blinded to minimize bias.
Interventions
The intervention group was asked to use the ePRO-CTCAE app in addition to the usual clinical practice. The ePRO-CTCAE app was designed to help cancer patients monitor their symptoms anytime and anywhere during chemotherapy. The application includes symptom monitoring questionnaires from the PRO-CTCAE-K, which is a valid and reliable measurement of cancer-related symptoms [26]. The app provided prespecified questionnaire sets depending on different types of cancer, which were determined by referring to a publication by the U.S. National Cancer Institute and expert opinions [27] (Supplementary Table S1). Research coordinators provided printed material, highlighting the importance of patient participation in the ePRO-CTCAE app [27]. The material consisted of the definition and examples of PRO, the clinical benefits of PRO, and practical tips on how to report PRO. The coordinator installed the app on the patients’ smartphones, educated the patients on how to use the ePRO-CTCAE app, provided a user manual, and asked the patients to complete an initial entry to the ePRO-CTCAE app. The researchers provided additional information if the patients had problems using the app.
The participants were instructed to complete a mobile symptom questionnaire from home using their mobile device every 7 days for 8 weeks. Reminders were sent weekly via the app on that day. If patients missed the reporting, additional reminders were sent for the first 2 days (twice a day) through the app, and on the 3rd day, the research coordinators called the patient to determine whether the patient had any technical or physical problems using the app. Once the patients reported symptoms, the ePRO-CTCAE app provided graphical summaries to help them understand their health status (Supplementary Fig. S1). Simultaneously, the reported data were automatically transferred to a web dashboard, where physicians could directly access them during practice. Additionally, we provided the physician with a printed summary report of the patient-input symptoms using the ePRO-CTCAE app (Supplementary Fig. S2). As the purpose of this study was to evaluate the effect of ePRO on symptom management participation, the web dashboard did not include an alert system for physicians. However, physicians were able to log in to the dashboard and view the severity of symptoms which were highlighted in red if they were willing to.
The patients in the control group received the usual clinical practice and the same educational material as the intervention group, which included information regarding the importance of patient participation in reporting PRO. During the study period, they could use a symptom diary to record their symptoms. We applied the same conditions to both the intervention and control groups, except for the ePRO-CTCAE app.
Outcomes
The primary outcome was the improvement in patient participation in symptom management during anticancer therapy. As there was no specific tool to assess patient participation in symptom management, an expert group consisting of two physicians, two oncology nurses, and three behavior scientists developed the questionnaire based on a literature review. The initial questions were developed according to the Patient Feedback Form [28]. Considering that patients’ health behavior can be affected by their beliefs, knowledge, and physical or social environments, we included barriers to participation in symptom management [28,29,30]. Among the 16 items of the initial questionnaire, exploratory factor analysis and confirmatory factor analysis yielded 10 items (Supplementary Text S1) with an acceptable model fit (Cronbach’s α = 0.77). The assessment comprised six items on the importance of participation and four items on the barriers to participation in symptom management. Respondents were instructed to indicate the importance of participation on a 4- or 5-point Likert scale (1 = Not at all, 2 = A little, 3 = Quite a bit, 4 = Very much; 1 = Never, 2 = Rarely, 3 = Sometimes, 4 = Often, 5 = Always) and the barriers to using the ePRO-CTCAE app on a binomial scale (0 = no, 1 = yes). However, because scoring data in this manner resulted in multiple violations of the assumption of multivariate normality, responses were scored as 0 for never, a little, and a lot and 1 for very much. Similarly, responses related to frequency were scored as 1 for Always and 0 for the others. The total scores ranged from 0 to 10.
The secondary outcomes were HRQoL and resource utilization. HRQoL was measured using the last two questions of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC-QLQ-C30). These questions were scored on a 7-point scale. According to the EORTC scoring manual [31], all scales must be transformed into scores from 0 to 100. A higher score indicates a better quality of life. Utilization of resources was measured by asking whether the patients had any emergency room (ER) visits, unplanned outpatient visits, or unplanned hospitalizations due to symptoms or treatment toxicities during the study period. Patients in both the intervention and control groups completed the repeat questionnaires at baseline and after the intervention (8 weeks after baseline). A baseline survey was conducted using a paper-based questionnaire, and the post-survey was conducted 8 weeks after the baseline survey using a web-based questionnaire through a popular survey tool, SurveyMonkey® (https://www.surveymonky.com).
In addition to the outcome measures, the patients provided sociodemographic information at baseline, including marital status, education level, monthly income, residence, health literacy, and digital health literacy. Health literacy was evaluated using the Newest Vital Sign (NVS), which asks six questions regarding nutrition labels on an ice cream container [32]. According to the scoring manual of the NVS, scores of 4 to 6 indicate adequate literacy, and less than 4 indicates limited literacy. Digital health literacy was evaluated using the 20-item questionnaire developed by Yoon [33]. The patients were asked to answer whether they could perform the task by themselves (yes, no, or do not know). The scores ranged from 0 to 20, with 11 to 20 indicating adequate digital health literacy and less than 11 indicating limited digital health literacy. Clinical information, including age, stage at diagnosis, and treatment modalities, was obtained from electronic medical records.
Statistical methods
The primary outcome was patient participation in symptom management after the intervention. As no previous study had been conducted that determined the sample size, we generated a hypothesis based on a similar intervention study. According to a meta-analysis study, the desired effect size (Cohen’s d) was at least 0.4 to present the impact of the intervention itself, which aims to change the participants’ perceptions, attitudes, or behaviors [34]. Assuming a probability of type I error (α) of 0.05 (two-sided), a power of 90%, and a 15% loss to follow-up, we estimated that 222 patients (151 patients in the intervention arm and 71 patients in the control arm) were needed to present a medium effect size (d = 0.5) in the primary outcome after the intervention. The intervention group was expected to exhibit better-engaged behavior in the context of symptom management than the control group.
All analyses were performed using the intention-to-treat approach. T-tests were used for comparing the primary outcomes of patient participation and secondary outcomes at each time point between the intervention and control groups. Linear mixed models were used for calculating the differences between the baseline and follow-up in the longitudinal data analysis.
In addition, stratified analyses were performed for evaluating the impact of the intervention on participation in symptom management post-intervention in prespecified subgroups defined by age (< 60 and ≥ 60 years); sex (male and female); marital status (unmarried and married); educational level (< college and ≥ college); health literacy (adequate and limited); digital literacy (adequate and limited); types of cancer (lung, breast, head and neck, esophageal, and gynecologic cancers); Surveillance, Epidemiology, and End Results (SEER) stages (in situ/localized, regional, distant, and unknown); and treatment modalities (chemotherapy or radiotherapy alone and combination therapies). All statistical analyses were performed using STATA 16.0 (Stata Corp LP, College Station, Texas, USA) and R 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined using a 95% confidence interval (CI) and a P-value < 0.05.
Results
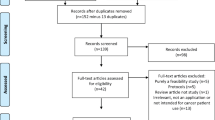
Between October 2020 and January 2021, 488 patients were screened for eligibility, and 378 met the eligibility criteria. Among the 378 eligible patients, 222 (58.7%) agreed to participate in the study, and they were randomly assigned to either the intervention (n = 147) or control (n = 75) group (Fig. 1). Among those who rejected the study provided, 83 were not interested in this study, 48 were too busy, and 25 were too ill. Five patients in the intervention group and one patient in the control group withdrew before the post-intervention survey. One patient in the control group died during the study period. The average age of the study participants was 55.9 years, and 38% were male. The patient characteristics were well balanced between the two treatment groups (Table 1).
Among the 221 remaining patients, 142 patients in the intervention group and 71 patients in the control group completed the post-intervention survey (8 weeks after baseline), and they were finally included in the analysis. On average, 88.6% of the patients in the intervention group (range, 74.6–93.1%) completed symptom reports with the ePRO-CTCAE app during the 8-week intervention period. A total of 56.0% of patients (79 of 142) in the intervention group completed all eight symptom reports.
At baseline, the intervention and control groups reported similar scores in patient participation in symptom management during anticancer therapy (mean scores of 8.2 vs. 8.2; P = 0.89, respectively; Table 2). As the primary outcome, the intervention group reported better outcomes in patient participation in symptom management than the control group at 8 weeks (mean scores of 8.5 vs. 8.0; P = 0.01). The group-by-time interaction effect was significant for patient participation in symptom management (P for interaction = 0.02; Fig. 2).
The positive association between the intervention and higher participation in symptom management was consistent in most prespecified subgroups analyzed, including old age, low digital literacy, and low education level (P-values for interaction > 0.05; Fig. 3).
Furthermore, the intervention group was more likely to have a good quality of life (35.9 vs. 33.8%) and less likely to experience hospitalization (33.8 vs. 40.8%), ER visits (19.7 vs. 22.5%), and unexpected clinical visits (19 vs. 23.9%) than the control group (Table 3). There were no harmful or unintended effects in either the intervention or control groups.
Discussion
In this randomized controlled trial, cancer patients using the ePRO-CTCAE app showed better participation in symptom management than those in the control group. The association between the use of the ePRO-CTCAE app and improved patient participation in symptom management was similar in most subgroup analyses, including in participants of older age, lower education, and lower digital literacy level. Even though it was not statistically significant, patients with the ePRO-CTCAE app tended to exhibit a better quality of life and experience fewer hospitalizations, ER visits, and other unexpected clinical visits than the control group.
In this trial, patients who used the ePRO-CTCAE app were more likely to participate in symptom management than those in the control group. This finding can be supported by other previous studies [15,16,17, 35]. This might be because the ePRO-CTCAE app provides facilitating conditions for both patients and their physicians in the context of symptom management. Real-time monitoring via the app may help patients become more aware of changes in their symptoms during treatment [16, 17]. Previous reports indicate that patients could better inform their providers regarding their health status [16, 17] and that they felt more comfortable initiating discussions with the medical team about their symptoms or other concerns [16]. It has also helped build a better rapport and break down barriers in communication between patients and clinicians [35]. Compared to the control group, the intervention group tended to show more positive perceptions and attitudes toward the importance of symptom reporting and self-efficacy.
Our findings showed that the positive association between the intervention and higher participation in symptom management was consistent with old age, low digital literacy, and a low education level, which is different from the results of previous studies. This appears to be possible because of the stable mobility environment, educational support for PRO and the ePRO-CTCAE app, convenience of the mobile app, and user-centric design of the app. The high mobility and accessibility of smartphones in Korea might contribute to patient participation in symptom management using the ePRO-CTCAE app. In addition, study participants in the intervention groups received education on PRO and instructions for using the app before the intervention. This pre-intervention support would resolve the technical barriers faced by patients, enabling them to use the ePRO-CTCAE app appropriately [36]. Over the 8 weeks, the overall compliance rate in the intervention group was nearly 90%, but only 56% of them completed all required symptom reports. In other words, 44% of them had at least one missed report during the study period. This might be associated with unfamiliarity with the ePRO-CTCAE system (self-monitoring system using the application). Although our mobile application had automatic reminders and notifications based on a user-centered design, it might take time for patients to use it regularly [36]. Or patients might not be able to report symptoms on a certain day because of their health condition [37]. According to feasibility studies of the web- and desktop-based symptom monitoring, feeling unwell or sick was one of the main barriers faced by patients when completing their symptom reports both at outpatient clinics and at home [11, 37,38,39]. It would be necessary to conduct additional studies to identify ways to improve the compliance rate of using a digital-based self-monitoring system.
In our study, ePRO-CTCAE app contributed to lowering their barriers regarding symptom reporting. However, 90% of the intervention group still felt difficulties in recalling and describing their symptoms. This might be due to 7-day recall period. In our study, we asked patients to report their symptoms every 7 days, and some patients might experience difficulties to remember things in past 7 days. Although it would be complicated to interpret the data, applying ecological momentary assessment would be more useful to remove these barriers [40].
The results of our study could not confirm the benefits of the ePRO-CTCAE app in terms of HRQoL and unplanned clinical visits, including ER visits and hospital readmissions. This may be because of the small sample size. According to a randomized controlled trial in 766 cancer patients, routine surveillance of symptoms had an impact on reducing the decline in HRQoL and decreasing the frequency of ER admission or hospitalization [13]. Our study was performed with a relatively small population to present a sufficient effect size compared with the previous study.
This study has several limitations. First, no blinding was performed in this study because of the nature of the intervention. Hence, the physicians involved in this study may have paid attention to the patients in the intervention group, and their attention could be potentially associated with patient experience regarding the intervention. To avoid this potential bias, we sought to ensure the blinding of the outcome assessors. However, this can also be considered an intervention element. Raising physicians’ awareness of patient problems is one of the many purposes of using PRO measures in routine clinical practice [41, 42]. Second, patient participation was collected using PRO, which could be influenced by the participants’ characteristics or social desirability. However, all participants were randomly allocated, and we performed a subgroup analysis, and the results were consistent with those of the main analysis. Third, to assess the cancer patients’ participation, a questionnaire designed by the researchers was used because there was no specific tool for it at the time. However, we confirmed the internal consistency of our questionnaire (Cronbach’s α = 0.77). Lastly, this study was conducted at a tertiary cancer hospital in South Korea, which means that the findings may not be generalizable to patients in different settings or other countries. There can be potentially selection bias due to the exclusion of iPhone users in this study. However, in South Korea, about 73% of smartphone owners is using Android-based mobiles [43]. Nearly 90% of smartphone owners in their 40 s and above are using Android-based mobiles [44]. Thus, the impact of selection bias due to the exclusion may be weak given that the average age of cancer patients is relatively older.
In conclusion, we confirmed that the ePRO-CTCAE app can help patients participate in symptom management, especially in outpatient-based cancer treatment where patients are expected to take responsibility for self-symptom management would be helpful. Our findings suggest that digital-based tools, such as our ePRO-CTCAE app, can empower patients to take a more active role in their treatment. Healthcare providers can further improve symptom management by discussing symptoms with patients using their symptom reports, even in an outpatient-based treatment setting.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Code availability
The codes that support the findings of this study are available on request from the corresponding author.
Abbreviations
- EORTC-QLQ-C30:
-
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
- ePRO:
-
Electronic PRO symptom monitoring
- ER:
-
Emergency room
- HRQoL:
-
Health-related quality of life
- NVS:
-
Newest Vital Sign
- PRO:
-
Patient-reported outcome
- PRO-CTCAE:
-
Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events
- SEER:
-
Surveillance, Epidemiology, and End Results
References
Deshields TL, Potter P, Olsen S et al (2014) The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Support Care Cancer 22:1089–1096
Dilalla V, Chaput G, Williams T et al (2020) Radiotherapy Side Effects: Integrating a Survivorship Clinical Lens to Better Serve Patients. Curr Oncol 27:107–112
Kang D, Kim S, Kim H et al (2023) Surveillance of Symptom Burden Using the Patient-Reported Outcome Version of the Common Terminology Criteria for Adverse Events in Patients With Various Types of Cancers During Chemoradiation Therapy: Real-World Study. JMIR Public Health Surveill 9:e44105. https://doi.org/10.2196/44105
Kolb NA, Smith AG, Singleton JR et al (2018) Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer 26:1607–1615. https://doi.org/10.1007/s00520-017-3970-7
Tallon B, Blanchard E, Goldberg LJ (2010) Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol 63:333–336
Cohen L, De Moor CA, Eisenberg P et al (2007) Chemotherapy-induced nausea and vomiting—incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Stillman M, Cata JP (2006) Management of chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep 10:279–287
Cheung YT, Chan A, Charalambous A et al (2022) The use of patient-reported outcomes in routine cancer care: preliminary insights from a multinational scoping survey of oncology practitioners. Support Care Cancer 30:1427–1439. https://doi.org/10.1007/s00520-021-06545-7
Gandrup J, Ali SM, McBeth J et al (2020) Remote symptom monitoring integrated into electronic health records: A systematic review. J Am Med Inform Assoc 27:1752–1763. https://doi.org/10.1093/jamia/ocaa177
Howell D, Molloy S, Wilkinson K et al (2015) Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 26:1846–1858. https://doi.org/10.1093/annonc/mdv181
Nguyen H, Butow P, Dhillon H et al (2021) A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci 68:186–195. https://doi.org/10.1002/jmrs.421
Peyroteo M, Ferreira IA, Elvas LB et al (2021) Remote monitoring systems for patients with chronic diseases in primary health care: systematic review. JMIR Mhealth Uhealth 9:e28285
Basch E, Deal AM, Kris MG et al (2016) Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 34:557–565. https://doi.org/10.1200/JCO.2015.63.0830
Hernandez Silva E, Lawler S, Langbecker D (2019) The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: a systematic review. J Cancer Surviv 13:97–107. https://doi.org/10.1007/s11764-018-0730-8
Maguire R, McCann L, Kotronoulas G et al (2021) Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ 374:n1647–n1647. https://doi.org/10.1136/bmj.n1647
Takeuchi EE, Keding A, Awad N et al (2011) Impact of patient-reported outcomes in oncology: a longitudinal analysis of patient-physician communication. J Clin Oncol 29:2910–2917
Yang LY, Manhas DS, Howard AF et al (2018) Patient-reported outcome use in oncology: a systematic review of the impact on patient-clinician communication. Support Care Cancer 26:41–60. https://doi.org/10.1007/s00520-017-3865-7
Denis F, Lethrosne C, Pourel N et al (2017) Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. J Natl Cancer Inst 109. https://doi.org/10.1093/jnci/djx029
Basch E, Deal AM, Dueck AC et al (2017) Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 318:197–198. https://doi.org/10.1001/jama.2017.7156
Rocque GB (2022) Learning From Real-world Implementation of Daily Home-Based Symptom Monitoring in Patients With Cancer. JAMA Netw Open 5:e221090–e221090. https://doi.org/10.1001/jamanetworkopen.2022.1090
All Party Parliamentary Group on Global Health (2014) Patient empowerment: for better quality, more sustainable health services globally. International Alliance of Patients’ Organizations. https://www.iapo.org.uk/news/2014/may/22/report-patient-empowerment-better-quality-more-sustainable-health-services-globally. Accessed 12 Oct 2022
Tang PC, Lansky D (2005) The missing link: bridging the patient–provider health information gap. Health Aff 24:1290–1295
Dedding C, Van Doorn R, Winkler L et al (2011) How will e-health affect patient participation in the clinic? A review of e-health studies and the current evidence for changes in the relationship between medical professionals and patients. Soc Sci Med 72:49–53
Marzban S, Najafi M, Agolli A et al (2022) Impact of Patient Engagement on Healthcare Quality: A Scoping Review. J Patient Exp 9:23743735221125440. https://doi.org/10.1177/23743735221125439
Maguire R, McCann L, Kotronoulas G et al (2021) Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ 374:n1647. https://doi.org/10.1136/bmj.n1647
Cho J, Yoon J, Kim Y et al (2019) Linguistic Validation of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events in Korean. J Global Oncol 5:1–10. https://doi.org/10.1200/JGO.18.00193
Dueck AC, Mendoza TR, Mitchell SA et al (2015) Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1:1051–1059. https://doi.org/10.1001/jamaoncol.2015.2639
Tolstrup LK, Pappot H, Zangger G et al (2018) Danish translation, cultural adaption and initial psychometric evaluation of the patient feedback form. Health Qual Life Outcomes 16:77. https://doi.org/10.1186/s12955-018-0900-4
Kühnel MB, Marchioro L, Deffner V et al (2020) How short is too short? A randomised controlled trial evaluating short-term existential behavioural therapy for informal caregivers of palliative patients. Palliat Med 34:806–816. https://doi.org/10.1177/0269216320911595
McCutchan G, Wood F, Smits S et al (2016) Barriers to cancer symptom presentation among people from low socioeconomic groups: a qualitative study. BMC Public Health 16:1052. https://doi.org/10.1186/s12889-016-3733-2
Fayers P, Aaronson N, Bjordal K et al (2001) EORTC QLQ-C30 Scoring Manual EORTC Quality of Life Group. EORTC, Brussels
Weiss BD, Mays MZ, Martz W et al (2005) Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 3:514–522
Yoon J, Lee M, Ahn JS et al (2022) Development and Validation of Digital Health Technology Literacy Assessment Questionnaire. J Med Syst 46. https://doi.org/10.1007/s10916-022-01800-8
Hattie J (2008) Visible learning: a synthesis of over 800 meta-analyses relating to achievement (1st ed). Routledge. https://doi.org/10.4324/9780203887332
Sawesi S, Rashrash M, Phalakornkule K et al (2016) The Impact of Information Technology on Patient Engagement and Health Behavior Change: A Systematic Review of the Literature. JMIR Med Inform 4: e1. https://doi.org/10.2196/medinform.4514
Lee M, Kang D, Kim S et al (2022) Who is more likely to adopt and comply with the electronic patient-reported outcome measure (ePROM) mobile application? A real-world study with cancer patients undergoing active treatment. Support Care Cancer 30:659–668. https://doi.org/10.1007/s00520-021-06473-6
Cho Y, Zhang H, Harris MR et al (2021) Acceptance and Use of Home-Based Electronic Symptom Self-Reporting Systems in Patients With Cancer: Systematic Review. J Med Internet Res 23:e24638. https://doi.org/10.2196/24638
Snyder CF, Blackford AL, Wolff AC et al (2013) Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology 22:895–901. https://doi.org/10.1002/pon.3087
Baeksted C, Pappot H, Nissen A et al (2017) Feasibility and acceptability of electronic symptom surveillance with clinician feedback using the Patient-Reported Outcomes version of Common Terminology Criteria for Adverse Events (PRO-CTCAE) in Danish prostate cancer patients. J Patient-Reported Outcome 1:1. https://doi.org/10.1186/s41687-017-0005-6
Thong MSY, Chan RJ, van den Hurk C et al (2021) Going beyond (electronic) patient-reported outcomes: harnessing the benefits of smart technology and ecological momentary assessment in cancer survivorship research. Support Care Cancer 29:7–10. https://doi.org/10.1007/s00520-020-05648-x
Basch E, Dueck AC, Rogak LJ et al (2018) Feasibility of implementing the patient-reported outcomes version of the common terminology criteria for adverse events in a multicenter trial: NCCTG N1048. J Clin Oncol 36:3120. https://doi.org/10.1200/JCO.2018.78.8620
Basch E, Iasonos A, McDonough T et al (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909. https://doi.org/10.1016/S1470-2045(06)70910-X
Statcounter; (2023) Mobile Operating System Market Share Republic Of Korea. Statcounter. https://gs.statcounter.com/os-market-share/mobile/south-korea/#monthly-202008-202101. Accessed 03 Mar 2023
Park EJ (2021) Old dudes love Galaxy phones, Samsung bummed. Korea JoonAng Daily. https://koreajoongangdaily.joins.com/2021/06/20/business/tech/Galaxy-Z-Flip-Apple/20210620070100647.html. Accessed 03 Mar 2023
Funding
This research was supported by a grant (19182MFDS426) from Ministry of Food and Drug Safety in 2020.
Author information
Authors and Affiliations
Contributions
Conceptualization: All authors.
Methodology: All authors.
Investigation: M Lee, E Kang, S Kim, and Y Kim.
Data Curation: M Lee, D Kang, E Kang, S Kim, and Y Kim.
Formal Analysis: M Lee, D Kang.
Visualization: M Lee, D Kang.
Writing – Original Draft Preparation: M Lee and D Kang.
Writing – Review & Editing: All authors.
Supervision: J Cho.
Resource: JS Ahn, SH Park, YY Lee, D Oh, and JM Noh.
Funding Acquisition: J Cho.
M Lee, Mangyeong Lee; D Kang, Danbee Kang; E Kang, Eunjee Kang; S Kim, Sooyeon Kim; Y Kim, Youngha Kim; JS Ahn, Jin Seok Ahn; SH Park, Se Hoon Park; YY Lee, Yoo-Young Lee; D Oh, Dongryul Oh; JM Noh Jae Myung Noh; J Cho, Juhee Cho.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board of the Samsung Medical Center (SMC 2020–04-215–004).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflicts of interest
None declared.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mangyeong Lee and Danbee Kang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M., Kang, D., Kang, E. et al. Efficacy of the PRO-CTCAE mobile application for improving patient participation in symptom management during cancer treatment: a randomized controlled trial. Support Care Cancer 31, 321 (2023). https://doi.org/10.1007/s00520-023-07779-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07779-3