Abstract
Purpose
Exercise is the core element of rehabilitation for cancer patients. However, most of the patients’ exercise levels failed to meet the indicators recommended by the guidelines or even decreased. Therefore, this umbrella review aims to provide an overview of review articles addressing the evidence of interventions to promote physical activity behavior change and increase physical activity among cancer patients.
Methods
We searched nine databases from inception to 12 May 2022 to obtain systematic reviews and meta-analyses of interventions to promote physical activity among cancer patients. The AMSTAR-2 was used for the quality assessment.
Results
Twenty-six individual systematic reviews including 13 studies performed meta-analyses. A total of 16 studies’ designs were all in randomized controlled trial. Most reviews included studies that were mainly delivered in home settings. The most frequent and mean duration of the interventions was 12 weeks. Interventions mainly included electronic, wearable health technology-based, behavior change techniques (BCTs), and theory-based strategies.
Conclusions
Electronic, wearable health technology-based, BCTs, and theory-based interventions were effective and feasible in promoting physical activity in cancer survivors. Clinical practitioners should take corresponding intervention measures according to the characteristics of patients in different groups.
Implications for cancer survivors
Future research may benefit cancer survivors by more comprehensively applying electronic, wearable health technology-based, BCTs, and theory-based interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
With socio-economic development and the prevalence of various cancer risk factors, the burden of cancer morbidity and mortality worldwide is increasing rapidly [1, 2]. In 2020, there are an estimated 1930 million new cancer cases and nearly 10 million cancer deaths worldwide [1]. Cancer patients have a higher cure rate and survival rate under the popularization of early screening and the progress of medical technology. Exercise is one of the vital measures for rehabilitation of many chronic diseases, improving the quality of life and reducing mortality [3, 4]. At the same time, exercise has a positive impact on anxiety, depression, fatigue, and quality of life decline caused by cancer or treatment [5]. Therefore, multidisciplinary cancer teams should encourage cancer patients to exercise.
Aerobic exercise and resistance exercise are safe, feasible, and effective in cancer patients during and after adjuvant therapy [6, 7]. According to the exercise guide for cancer patients, cancer patients should gradually resume their daily activities after surgery and recommended at least 150 min of aerobic exercise and 2 ~ 3 times of resistance training per week [5]. However, few patients can achieve this index. A 1-year study by Arem et al. [8] found that only 33% of women reached 150 min of exercise per week, suggesting that the physical activity (PA) level of breast cancer patients during treatment was reduced, and they also had less activity in the late stage of treatment. Therefore, after excluding exercise contraindications, when providing exercise advice and intervention for patients, attention should be paid to the maintenance or improvement of exercise compliance of patients to alleviate related symptoms and improve their quality of life of patients.
At present, more systematic evaluations focus on intervention measures for promoting exercise in cancer patients. Behavioral change techniques are commonly used to improve patient compliance. Behavioral change strategies, such as pedometers, motivational interviews, and materials, can improve patient compliance with exercise and increase their exercise level [9]. Lopez et al. [10] reported that high-intensity resistance exercise and low-intensity resistance exercise are equally effective in patients with prostate cancer, and low exercise doses may help to reduce movement disorders and enhance compliance. The purpose of this umbrella review is to systematically summarize the interventions to promote physical activity behavior change and increase physical activity among cancer patients. More specifically, it aims to establish a clear cancer movement promotion plan (i.e., settings, length, measurement tools, and interventions), provide evidence to promote the movement of cancer patients, and can be used for clinical medical staff to develop exercise plans and provide suggestions for patients.
Methods and analysis
Protocol registration
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-P) [11] and registered at Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/; CRD42022316194).
Literature search strategy
Two authors (QL, YMD) independently and systematically searched the following databases, PubMed, Embase, MEDLINE, Cochrane Library, Scopus, CINAHL, Web of Science, OVID, and Research Square (gray literature) for studies published from inception to 12 May 2022.
The search strategy was developed following the participants, intervention, comparison, and outcomes (PICOs) components. The retrieval was carried out by the combination of keywords and free words (Supplemental File 1). Keywords were adjusted across databases. And we re-runned it before the final analysis (July 18) to ensure that there was no new research.
Inclusion/exclusion criteria for study selection
The inclusion criteria for this umbrella review according to PICOS format are as follows: (1) types of participants, cancer patients either completed or undergoing treatment; (2) types of interventions, any interventions that may promote or maintain physical activity behavior change and adherence, including but not limited to the motivational strategies, eHealth interventions, and behavior change techniques; (3) types of comparison, compares the intervention to an alternative intervention or usual care; (4) types of outcome, the primary outcome was physical activity behavior (self-reported or objectively measured) and secondary outcomes were including but not limited to physical activity adherence, self-efficacy, physical function, and quality of life; (5) types of studies, systematic reviews and meta-analyses of a quantitative or qualitative study. Criterion (5), systematic reviews that include qualitative and quantitative studies, aims to enrich the evidence.
Studies were excluded if they (1) reported the efficacy of a physical activity intervention that did not involve physical activity level or adherence or (2) were available as a conference abstract only.
Selection of studies and data extraction
Importing all search results into EndNote X9, title and abstract screening were conducted concurrently by two investigators trained in evidence-based courses (e.g., training in theories, principles, and methods of evidence-based medicine). If the title and abstract lack sufficient information to make a decision, the article was carried forward to the full-text screening stage. Full texts were obtained and screened independently by two investigators according to the inclusion and exclusion criteria of the literature.
The included literature was extracted after the literature quality evaluation by the self-designed standardized table to extract data; the main extracted content are as follows: title, first author, publication year, objective, study designs, number of studies included, analysis method, quality assessment tools, population, intervention, outcome measure, etc. Two researchers did data extraction independently, and inconsistencies were resolved by discussion.
Methodological quality assessment
The methodological quality of the included systematic reviews and meta-analyses was assessed using AMSTAR-2 [12], which includes 16 items. According to the instrument, two reviewers classified the results of included systematic reviews as high, moderate, low, and critically low. If the study has no or one non-critical weakness, we appraised it as high; if more than one non-critical weakness, we appraised it as moderate; if one critical flaw with or without non-critical weakness, we appraised it as low; and if more than one critical flaw with or without non-critical weakness, we appraised it as critically low.
Our study was founded on the concept of evidence from multiple sources. Included studies had interventional and qualitative studies. At the same time, the interventions and outcome indicators of this study are diverse, and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) is not applicable. Therefore, the evidence was pre-ranking using the Joanna Briggs Institute evidence pre-ranking system [13]. According to the type of included studies, it is divided into five levels from level 1 to 5.
Data analysis
Due to the factors such as different intervention techniques, different application times, and different research objects, this study only conducts a descriptive analysis of the included studies.
Results
Literature selection
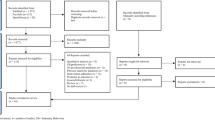
The initial search resulted in 1129 records through database searching; after the removal of duplicates, 820 records remained. Of these, excluded after title, abstract, and full-text screening according to the selection criteria, resulting in 26 articles. (Fig. 1).
Study characteristics
Overall, the 26 systematic reviews included 67,477 breast cancer patients from 8 countries; included studies were conducted in 8 different countries, mainly in the USA (7/26) and the UK (6/26). Sixteen of these study designs were all in randomized controlled trial. Thirteen studies performed meta-analyses. For the evaluation of the risk of bias, 18 of included studies used the Cochrane risk of the bias assessment tool, 2 used the PEDro scale, 2 used the EPHPP tool, 3 used other tools, and 1 was unclear that not reported for evaluation. The cancer population involved in the study is mainly breast cancer patients. Eight reviews focused on breast cancer survivors only [9, 14-20], sixteen reviews involved survivors with different types of cancer [21-36], and the remaining 2 reviews targeted survivors of colorectal cancer [37, 38] and pediatric cancer. Participants were completed cancer treatment or on remission phase in 10 reviews [9, 14, 15, 17-21, 23, 38], and the remaining reviews involved participants undergoing or completed cancer treatment [16, 22, 24-37]. The intervention measures to promote the movement of cancer patients include theoretical guidance, behavioral change technology, electronic technology intervention, and provision of counseling, print materials, etc. More detail on the basic characteristics of the included literature is shown in Table 1, and PICO information on included studies can be found in Table S1.
Methodological quality and evidence grade evaluation
The methodological quality evaluation results showed that 1 study was high, 10 were intermediate, 9 were low, and 6 were very low. Among the 7 key items of AMSTAR 2 quality evaluation, item 2 and item 15 have significant defects; the non-critical items with obvious defects are item 3 and item 10 (details in Figure S1). The results of the level of evidence evaluation showed that 16 articles were ranked as 1a, that is, systematic reviews of multiple RCTs; 10 articles were ranked as 1b, that is, systematic reviews of multiple RCTs and other interventional studies.
Summary of exercise promotion measures (Table 2)
Intervention settings
Intervention settings in our reviews included studies that included group, one on one, home, clinic or research-setting, and center-based. Most reviews included studies that were mainly delivered in home settings [9, 15, 19, 20, 23-25, 27-29, 38]. Some reviews that delivered in a variety of settings included a combination of supervised and home-based exercise, supervised combined with unsupervised [14, 16, 19, 26, 30]. In reviews that focused on a single intervention setting, all were conducted in a home setting [18, 33]. Home-based exercise usually has behavioral support, including information feedback, ongoing interaction, or counseling with the research team. Five reviews did not report the setting [17, 32, 34, 36, 37]. Interventions that included a home-based training component seemed more effective than clinics or gyms in a meta-analysis of 27 studies (β = 0.261; 95% CI = 0.135, 0.386; P ≤ 0.001) [23]. And the review by Rossi et al. [25] indicated that home-based combined center-based interventions may increase physical activity more than home-based programs alone among overweight and obese female cancer survivors.
Intervention length
The intervention length varied from 2 weeks to 4.78 years. The most frequent and mean duration of the interventions was 12 weeks [14, 18, 20, 29, 30, 32, 34]. Four reviews included the length of reporting ranging from a minimum of 12 weeks [16, 19, 25, 33], four reviews included the length of interventions ranging to 12 months [23, 24, 35, 36]. The studies included a one-time recommendation, or follow-up during intervention and post-intervention, as Grimmett et al. [27] included a physical activity behavior follow-up assessment ranging from 3 months post-intervention to 5 years post-intervention. The review by Singh et al. [36] indicated that interventions lasting 12 weeks (SMD = 0.73) were greater at increasing MVPA compared with interventions < 12 weeks (SMD = 0.19; x2 = 4.78, p = 0.03). And interventions are effective for a moderate increase in physical activity at least 3 months after the intervention (interventions detail in Table S1)[27].
Physical activity measures
Most studies used self-reported measures to assess the patients’ physical activity behavior. Nineteen reviews (73.07%) [14, 15, 17, 19, 21-25, 27, 28, 30-35, 37, 38] involved subjective PA measures; the subjective PA measurement tools used were mainly the Godin Leisure Time Exercise Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), 7-day physical activity recall (7-DPARQ), Godin Leisure score index, Scottish physical activity questionnaire (Scot-PASQ), the Short Questionnaire to Assess Health Enhancing Physical Activity (SQUASH), 7-day PA and Community Health Activities Model Program for Seniors (CHAMPS), etc. And some studies used a self-log method. Fourteen reviews (53.84%) [14, 17-19, 23-27, 32-35, 38] involved objective PA measures (i.e., accelerometers, Fitbit, and pedometers). The objective measurement tools are small and light; these can be worn on the waist or wrist during waking hours. Seven reviews did not clearly report the measures [9, 16, 20, 23, 29, 34, 36].
Types of interventions and their effectiveness on physical activity (Table S1)
Electronic interventions
Investigating the effect of electronic interventions on cancer survivors were 34.61% (n = 9) of the total included studies [17, 18, 22, 24, 28, 31, 32, 34, 36, 38]. Of these, seven studies aimed at the effect of electronic health (eHealth) on exercise levels in cancer patients [17, 22, 24, 31, 32, 34, 38]. eHealth uses technologies including telephones, websites, email, and mobile health (mHealth) technologies. The majority of studies (6/7) [17, 22, 24, 32, 34, 38] reported that eHealth interventions were effective and feasible in promoting physical activity in cancer survivors. eHealth resulted in a significant increase in MVPA minutes/week (MD = 41; 95% CI = 12, 71; p = 0.006) in a meta-analysis that included 5 RCTs and 2 pre-post studies [22]. Compared with the control interventions, eHealth technology provides communication (including real-time, automated reminders) and feedback.
Three studies were included which aim to examine the feasibility of digital activity trackers in cancer survivors and their effects on activity levels. And activity trackers were the most commonly used mHealth technology [34], and interventions based on physical activity trackers and pedometers usually include BCTs or theory-based interventions [22, 36]. In the included studies, wearable physical activity trackers mainly include Fitbit One, Polar A360/M400, GT3X + ActiGraph, ActivPAL, and Garmin Vivofit smartwatches. Four reviews [18, 28, 31, 34] found that wearable health technology–based physical activity interventions are effective in improving physical activity, and health-related outcomes in individuals with cancer. The review by Khoo et al. [34] indicated there is strong evidence that mHealth includes an individual exposure component to increase moderate intensity PA in cancer survivors. And physical activity tracker and pedometer-based interventions had moderate-to-large effects (all p < 0.05) on the duration of moderate-intensity physical activity (SMD = 0.87; 95% CI = 0.43, 1.32), MVPA (SMD = 0.61;95% CI = 0.36,0.86), total physical activity (SMD = 0.62; 95% CI = 0.39, 0.84), and daily steps (SMD = 0.54; 95% CI = 0.30, 0.78) in a meta-analysis of 15, 16, and 19 studies, respectively [36]. Subgroup analysis by Singh et al. [36] showed that baseline counseling (compared to no baseline counseling taken) and theory-based were effective in increasing PA levels, respectively.
Behavior change techniques and theory-based interventions
Among the included studies, five studies were limited to behavioral change techniques (BCTs) and theory-based interventions [9, 19, 20, 23, 25], and five studies related to integrated interventions [14, 26, 30, 35, 37]. BCTs in the included studies were seven [9, 14, 23, 26, 30, 35, 37]. BCTs had a significant effect on PA levels in a meta-analysis of 30 studies (g = 0.28; 95% CI = 0.18, 0.37; I2 = 54.29%)[23]. Motivational strategies improved MVPA duration in a meta-analysis of 10 studies (SMD = 0.55; 95% CI = 0.30, 0.79; I2 = 51.4%) [20]. The most commonly used BCTs were goal setting (behavior), goal setting (outcome), action planning, habit reversal, instruction on how to perform the behavior, self-monitoring, eliciting social support, positive reinforcement, and problem-solving. And effect sizes did not increase meaningfully with the number of BCTs per intervention (β = 0.005; 95% CI = − 0.007, 0.017; P = 0.408) [23]. The BCTs prompts, reduce prompts, graded tasks, non-specific rewards, and social rewards were significantly associated with larger effects, while information about emotional consequences and social comparison was associated with smaller effects size [23]. Face-to-face behavior change counseling interventions improved physical activity behavior in a meta-analysis of 13 studies (SMD = 0.22; 95% CI = 0.11,0.33; p < 0.001; I2 = 6%) [35]. Supervision is most important for adherence [26], and involved supervised exercise sessions were associated with greater effectiveness in a meta-regression of 54 studies (B = 0.20, SE = 0.07, p = 0.005) [29].
Four reviews involved theory-based behavioral interventions [19, 25, 30, 37]. Most studies cited the social cognitive theory, the transtheoretical model, and the theory of planned behavior. Most reviews suggest that theory-based PA interventions can increase PA in cancer survivors. Theory-based PA interventions among colorectal cancer survivors improve physical activity in a meta-analysis of 8 studies (ES = 0.26; 95% CI = 0.13, 0.38; I2 = 16%) [37]. Behavior change theories had medium improvements in self-reported physical activity in a meta-analysis of 12 studies (SMD = 0.57; 95% CI = 0.33, 0.80; I2 = 67%) [19]. And there were smaller effect sizes for TPB-based interventions in a meta-analysis of 7 studies [23]. The included review based on 16 studies [20] showed that the combination of step trackers with counseling, printed materials, or motivational strategies based on behavioral change theory provided a consistently positive effect on adherence to self-directed PA among breast cancer survivors.
Psychoeducation
Dennett et al. [21] evaluated the effectiveness of psychoeducational interventions in physical activity behavior change among cancer survivors. This review of 2 randomized controlled trials demonstrated that adding psychoeducation to exercise rehabilitation programs had no additional benefit in improving physical activity behaviors or improving health outcomes in cancer survivors.
Other interventions
Five studies were included to assess the effectiveness of interventions in promoting physical activity [15, 16, 27, 29, 38]; interventions in included studies were workshops, group exercise, walking, behavioral counseling, home-based/group-supervised exercise classes, counseling, and group discussions, printed materials and pedometer, etc. Most studies use a combination of interventions for exercise promotion intervention. Reviews indicate that both group and individual PA interventions for individuals have had positive outcomes. Too frequent direct supervision may not be better for physical activity levels. And greater contact time was associated with increased effectiveness in a meta-regression of 63 studies (B = 0.002, SE = 0.001, p = 0.008)[29]. Reasonably low-intensity interventions may be sufficient to induce lasting behavioral change in positive, young, well-educated, and white populations, but other populations may require more intensive support, especially older and physically constrained populations. Overweight or sedentary participants had larger effect sizes on PA levels in a meta-regression of 84 studies (B = 0.15, SE = 0.07, p = 0.03).
Adverse events
Seven of the included reviews [21, 24-26, 33, 36, 38] reported the adverse reactions of patients during exercise intervention (26.9%, n = 26), and 19 reviews [9, 14-20, 22, 23, 27-32, 34, 35, 37] did not report and mention on adverse events at all. The review of Cheung et al. [38] showed that no major adverse events occurred in the 8 studies involving pediatric cancer survivors. And the study by Haberlin et al. [24] including 10 studies involving eHealth used interventions showed that no adverse effects have been reported. Adverse events included minor joint injuries/soreness [25, 36], recurrence of previous tendinitis [33], plantar fasciitis [26, 36], and falls [36]. In the exercise intervention of overweight and obese women, there is a serious adverse event, a pelvic stress fracture, was reported [25].
Discussion
Exercise is one of the vital rehabilitation strategies for cancer patients. This umbrella review summarized evidence from systematic reviews and meta-analyses on interventions that improve exercise levels and maintain or improve exercise compliance in cancer patients. The main findings of our umbrella review were that (1) the most frequent and mean duration of the exercise interventions was 12 weeks, and 3 months post-intervention completion can be assessed and guided; (2) electronic, wearable health technology-based, BCTs, and theory-based interventions were effective and feasible in promoting physical activity in cancer survivors; promoting exercise requires a combination of interventions; (3) digital activity trackers were the BCTs with the highest potential to increase PA; (4) the BCTs prompts, reduce prompts, graded tasks, non-specific reward, and social reward were significantly associated with larger effects, while information about emotional consequences and social comparison were associated with smaller effects size; and (5) higher BMI was associated with higher intervention effects, and interventions targeting overweight or sedentary participants were particularly effective.
In this umbrella review, most of the interventions reported statistically significant results for PA outcome in cancer patients. Studies [17, 22, 24, 32, 34, 38] have shown that electronic interventions are expected to improve the level of exercise in cancer patients. Particularly, physical activity tracker and pedometer-based interventions had moderate-to-large effects on duration of moderate-intensity physical activity[36]. eHealth is used as an auxiliary tool for exercise intervention. Most studies [22, 34, 36] on eHealth usually include counseling, educational sessions, personal contact, and BCTs. And baseline counseling is necessary for cancer patients during exercise intervention [35, 36]. There is strong evidence for BCTs in increasing moderate-to-vigorous intensity PA among cancer patients. It is worth noting that not all technologies seem to be applicable, and the effect of intervention may not increase with the increase of the number of BCTs per intervention. BCTs combining with activity trackers is the highest potential to increase PA [23]. Most of the studies show the importance of interaction and behavioral intervention on the exercise behavior of cancer patients, and there are few studies considering the maintenance of exercise behavior after intervention [16, 18, 24, 27, 32]. At the same time, the importance of personal contact components was also found in this umbrella review, but the frequency of assessment or feedback in exercise intervention needs further study.
Although exercise has been advocated, cancer survivors face a series of factors that may impede or facilitate their participation in physical activity. The key barriers to exercise were treatment-related side effects, lack of time, and fatigue [39]. And the critical facilitators of exercise include improved physical health, improved mental well-being, gaining control, and the social benefits of exercise [39]. And fatigue is one of the most commonly reported side effects of cancer treatment [40, 41]. About 70% of cancer patients receiving chemotherapy or radiotherapy have cancer-related fatigue [41]. Many studies have shown that exercise can effectively alleviate the fatigue of patients while improving other adverse reactions, as well as improving physical function and quality of life [42, 43]. However, fatigue is also one of the main obstacle factors for patients’ exercise. Therefore, it is particularly important to improve the exercise level of patients through intervention measures. The study by Machado et al. [44] showed that exercise training is an effective intervention to reduce CRF, especially in patients receiving chemotherapy. Similar to previous studies [45-47], by guiding patients in time management; providing effective, time-saving, and personalized exercise programs; and supporting relevant electronic equipment and network platforms, patients can be promoted to exercise. However, most of the participants in the included systematic review were in the post treatment, and more evidence is needed for measures related to PA level improvement in cancer patients undergoing treatment.
To our best knowledge, this umbrella review is the first to summarize the interventions for promoting physical activity. In similar studies on different populations, most of the physical activity promotion studies focus on children; few studies focus on adolescents or the elderly [48, 49]. In our study, most interventions tend to be a combination of approaches, including electronic, wearable health technology-based, BCTs, theory-based, and combined interventions. Disease treatment seeks targeted intervention, and if exercise intervention can be targeted, it can also maximize the patient’s exercise level and exercise compliance. The study by Rossi et al. [25] found that among overweight and obese female cancer survivors, home-based integration of center-based physical activity interventions may improve patient activity levels more than home-based programs alone. Interventions are more effective in overweight or sedentary patients than in overweight and obese women. Supervision of sedentary patients is the most important measure to improve compliance [26]. However, in terms of interventions for different symptoms, more research is needed to explore the better effectiveness of different interventions.
Limitation
The quality of the comprehensive review depends on the quality of the included reviews, which in turn depends on the quality of the primary study. Therefore, we need to consider the limitations of the study. First, moderate to high-grade studies were limited as assessed by the AMSTAR 2, mainly related to the study’s protocol design and reporting, and investigation and discussion of publication bias, a common mistake in most systematic reviews. Second, although the incidence of adverse events was low in the reported studies, the included reviews lacked monitoring of the adverse effects of exercise interventions. Finally, the details of the intervention still need further research, including the effect of duration and frequency on patients’ physical activity levels.
Conclusion
Oriented to positive health and modifiable factors, our study provides an overview of strategies used in the field of exercise promotion, clarifying the intervention setting, duration of intervention, and intervention strategies. It provides an evidence-based basis and reference for clinical practitioners who need rapid access to evidence related to the promotion of exercise in cancer patients. There is abundant evidence that eHealth, wearable health technology-based (combine with theory, BCTs, counseling, etc.), BCTs, and theory-based interventions were effective and feasible in promoting physical activity in cancer survivors. Faced with different groups of patients, such as overweight, sedentary behavior, the elderly, and limited physical activity, appropriate interventions should be adopted according to the characteristics of the population.
Data availability
We have full control of all systematic review data and can allow the journal to review, if requested.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Posadzki P, Pieper D, Bajpai R et al (2020) Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 20(1):1724. https://doi.org/10.1186/s12889-020-09855-3
Momma H, Kawakami R, Honda T et al (2022) Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med 56(13):755–763. https://doi.org/10.1136/bjsports-2021-105061
Segal R, Zwaal C, Green E et al (2017) Exercise for people with cancer: a clinical practice guideline. Curr Oncol 24(1):40–46. https://doi.org/10.3747/co.24.3376
Campbell KL, Winters-Stone KM, Wiskemann J et al (2019) Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51(11):2375–2390. https://doi.org/10.1249/MSS.0000000000002116
Singh B, Spence RR, Steele ML et al (2018) A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women With Stage II+ Breast Cancer. Arch Phys Med Rehabil 99(12):2621–2636. https://doi.org/10.1016/j.apmr.2018.03.026
Arem H, Sorkin M, Cartmel B et al (2016) Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the Hormones and Physical Exercise (HOPE) study. J Cancer Surviv Res Pract 10(4):654–662. https://doi.org/10.1007/s11764-015-0511-6
Hailey V, Rojas-Garcia A, Kassianos AP (2022) A systematic review of behaviour change techniques used in interventions to increase physical activity among breast cancer survivors. Breast Cancer (Tokyo, Japan) 29(2):193–208. https://doi.org/10.1007/s12282-021-01323-z
Lopez P, Galvão DA, Taaffe DR et al (2021) Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer (Tokyo, Japan) 28(1):16–24. https://doi.org/10.1007/s12282-020-01147-3
Honjo S, Mizunuma H, Soda M et al (1989) Effect of estrogen replacement therapy on the serum osteocalcin level in the postmenopausal and castrated women. Nihon Sanka Fujinka Gakkai Zasshi 41(10):1571–1576
Shea BJ, Reeves BC, Wells G et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 358(j4008.https://doi.org/10.1136/bmj.j4008
JBI Levels of Evidence and Grades of Recommendation Working Party (2014) JBI LEVELS OF EVIDENCE. https://jbi.global
Short CE, James EL, Stacey F et al (2013) A qualitative synthesis of trials promoting physical activity behaviour change among post-treatment breast cancer survivors. J Cancer Surviv 7(4):570–581. https://doi.org/10.1007/s11764-013-0296-4
Bluethmann SM, Vernon SW, Gabriel KP et al (2015) Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat 149(2):331–342. https://doi.org/10.1007/s10549-014-3255-5
Abdin S, Lavallée JF, Faulkner J et al (2019) A systematic review of the effectiveness of physical activity interventions in adults with breast cancer by physical activity type and mode of participation. Psychooncology 28(7):1381–1393. https://doi.org/10.1002/pon.5101
Dorri S, Asadi F, Olfatbakhsh A et al (2020) A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer (Tokyo, Japan) 27(1):25–46. https://doi.org/10.1007/s12282-019-00982-3
Blount DS, McDonough DJ, Gao Z (2021) Effect of wearable technology-based physical activity interventions on breast cancer survivors’ physiological, cognitive, and emotional outcomes: A systematic review. J Clin Med 10(9):2015. https://doi.org/10.3390/jcm10092015
Liu MG, Davis GM, Kilbreath SL et al (2021) Physical activity interventions using behaviour change theories for women with breast cancer: a systematic review and meta-analysis. J Cancer surviv Res Pract Online Publ. https://doi.org/10.1007/s11764-021-01104-9
Pudkasam S, Feehan J, Talevski J et al (2021) Motivational strategies to improve adherence to physical activity in breast cancer survivors: A systematic review and meta-analysis. Maturitas 152:32–47. https://doi.org/10.1016/j.maturitas.2021.06.008
Dennett AM, Shields N, Peiris CL et al (2017) Does psychoeducation added to oncology rehabilitation improve physical activity and other health outcomes? A systematic review. Rehabil Oncol 35(2):61–71. https://doi.org/10.1097/01.REO.0000000000000045
Roberts A, Fisher A, Smith L et al (2017) Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 11(6):704–719. https://doi.org/10.1007/s11764-017-0632-1
Finne E, Glausch M, Exner A-K et al (2018) Behavior change techniques for increasing physical activity in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res 10:5125–5143. https://doi.org/10.2147/CMAR.S170064
Haberlin C, O’Dwyer T, Mockler D et al (2018) The use of eHealth to promote physical activity in cancer survivors: a systematic review. Support Care Cancer 26(10):3323–3336. https://doi.org/10.1007/s00520-018-4305-z
Rossi A, Friel C, Carter L et al (2018) Effects of Theory-Based Behavioral Interventions on Physical Activity Among Overweight and Obese Female Cancer Survivors: A Systematic Review of Randomized Controlled Trials. Integr Cancer Ther 17(2):226–236. https://doi.org/10.1177/1534735417734911
Turner RR, Steed L, Quirk H et al (2018) Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 9(9):Cd010192. https://doi.org/10.1002/14651858.CD010192.pub3
Grimmett C, Corbett T, Brunet J et al (2019) Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int J Behav Nutr Phys Act 16(1):37. https://doi.org/10.1186/s12966-019-0787-4
Schaffer K, Panneerselvam N, PohLoh K et al (2019) Systematic review of randomized controlled trials of exercise interventions using digital activity trackers in patients with cancer. JNCCN J Natl Compr Cancer Netw 17(1):57–63. https://doi.org/10.6004/jnccn.2018.7082
Sheeran P, Abraham C, Jones K et al (2019) Promoting physical activity among cancer survivors: Meta-analysis and meta-CART analysis of randomized controlled trials. Health Psychol 38(6):467–482. https://doi.org/10.1037/hea0000712
Brunet J, Wurz A, Nader PA et al (2020) A systematic review summarizing the effect of health care provider-delivered physical activity interventions on physical activity behaviour in cancer survivors. Patient Educ Couns 103(7):1287–1301. https://doi.org/10.1016/j.pec.2020.02.002
Blackwood J, Rybicki K (2021) Outcomes of Telehealth-Delivered Physical Activity Programs in Adult Cancer Survivors: A Systematic Review. Rehabil Oncol 39(3):128–136. https://doi.org/10.1097/01.REO.0000000000000249
Ester M, Eisele M, Wurz A et al (2021) Current Evidence and Directions for Future Research in eHealth Physical Activity Interventions for Adults Affected by Cancer: Systematic Review. JMIR Cancer 7(3):1–27. https://doi.org/10.2196/28852
Ibeggazene S, Turner R, Rosario D et al (2021) Remote interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review and meta-analysis. BMC Cancer 21(1):308. https://doi.org/10.1186/s12885-021-07989-0
Khoo S, Mohbin N, Ansari P et al (2021) Mhealth interventions to address physical activity and sedentary behavior in cancer survivors: A systematic review. Int J Environ Res Public Health 18(11):5798. https://doi.org/10.3390/ijerph18115798
Meyer-Schwickerath C, Morawietz C, Baumann FT et al (2021) Efficacy of face-to-face behavior change counseling interventions on physical activity behavior in cancer survivors–a systematic review and meta-analysis. Disability and rehabilitation 1–16. Advance online publication. https://doi.org/10.1080/09638288.2021.1938247
Singh B, Zopf EM, Howden EJ (2022) Effect and feasibility of wearable physical activity trackers and pedometers for increasing physical activity and improving health outcomes in cancer survivors: A systematic review and meta-analysis. J Sport Health Sci 11(2):184–193. https://doi.org/10.1016/j.jshs.2021.07.008
Mbous YP, Patel J, Kelly KM (2020) A systematic review and meta-analysis of physical activity interventions among colorectal cancer survivors. Transl Behav Med 10(5):1134–1143. https://doi.org/10.1093/tbm/ibz176
Cheung AT, Li WHC, Ho LLK et al (2021) Physical activity for pediatric cancer survivors: a systematic review of randomized controlled trials. J Cancer Surviv Res Pract 15(6):876–889. https://doi.org/10.1007/s11764-020-00981-w
Clifford BK, Mizrahi D, Sandler CX et al (2018) Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer 26(3):685–700. https://doi.org/10.1007/s00520-017-3964-5
Bower JE (2014) Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609. https://doi.org/10.1038/nrclinonc.2014.127
Bower JE, Ganz PA, Desmond KA et al (2000) Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18(4):743–753. https://doi.org/10.1200/JCO.2000.18.4.743
Gebruers N, Camberlin M, Theunissen F et al (2019) The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support Care Cancer 27(1):109–122. https://doi.org/10.1007/s00520-018-4490-9
Ehlers DK, DuBois K, Salerno EA (2020) The effects of exercise on cancer-related fatigue in breast cancer patients during primary treatment: a meta-analysis and systematic review. Expert Rev Anticancer Ther 20(10):865–877. https://doi.org/10.1080/14737140.2020.1813028
Machado P, Morgado M, Raposo J et al (2022) Effectiveness of exercise training on cancer-related fatigue in colorectal cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 30(7):5601–5613. https://doi.org/10.1007/s00520-022-06856-3
Fox L, Wiseman T, Cahill D et al (2019) Barriers and facilitators to physical activity in men with prostate cancer: A qualitative and quantitative systematic review. Psychooncology 28(12):2270–2285. https://doi.org/10.1002/pon.5240
Michael M, Goble J, Hawk M et al (2021) Reported Barriers Impeding Adherence to a Physical Exercise Program in Patients With Breast Cancer: A Systematic Review. Rehabil Oncol 39(2):88–102. https://doi.org/10.1097/01.REO.0000000000000220
Lavallee JF, Abdin S, Faulkner J et al (2019) Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: A qualitative metasynthesis. Psychooncology 28(3):468–476. https://doi.org/10.1002/pon.4980
Craike M, Wiesner G, Hilland TA et al (2018) Interventions to improve physical activity among socioeconomically disadvantaged groups: an umbrella review. Int J Behav Nutr Phys Act 15(1):43. https://doi.org/10.1186/s12966-018-0676-2
Barbosa Filho VC, Minatto G, Mota J et al (2016) Promoting physical activity for children and adolescents in low- and middle-income countries: An umbrella systematic review: A review on promoting physical activity in LMIC. Prev Med 88:115–126. https://doi.org/10.1016/j.ypmed.2016.03.025
Acknowledgements
We would appreciate all the authors of the original meta-analysis of exercise promotion in cancer patients for their contributions.
Funding
This work was supported by the Scientific Research Project of the Shanghai Municipal Health Commission (grant no. 201940502), Pudong New Area Health System Discipline Construction Project (grant no. PWZxk2022-06), and Hospital Management Research Fund of Shanghai Hospital Association (grant no. K2022058).
Author information
Authors and Affiliations
Contributions
Qiu L and Ye MD (these authors contributed equally to this work and should be considered co-first authors): conception, design, literature search, formal analysis, project administration, writing—original draft. Tong Y: formal analysis, supervision, writing—review and editing. Jin YM: conception, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiu, Lin and Ye, Maodie contributed equally to this work and should be considered co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, L., Ye, M., Tong, Y. et al. Promoting physical activity among cancer survivors: an umbrella review of systematic reviews. Support Care Cancer 31, 301 (2023). https://doi.org/10.1007/s00520-023-07760-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07760-0